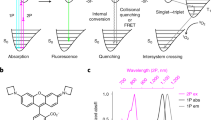
Abstract
Pushing the frontier of fluorescence microscopy requires the design of enhanced fluorophores with finely tuned properties. We recently discovered that incorporation of four-membered azetidine rings into classic fluorophore structures elicits substantial increases in brightness and photostability, resulting in the Janelia Fluor (JF) series of dyes. We refined and extended this strategy, finding that incorporation of 3-substituted azetidine groups allows rational tuning of the spectral and chemical properties of rhodamine dyes with unprecedented precision. This strategy allowed us to establish principles for fine-tuning the properties of fluorophores and to develop a palette of new fluorescent and fluorogenic labels with excitation ranging from blue to the far-red. Our results demonstrate the versatility of these new dyes in cells, tissues and animals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lavis, L.D. & Raines, R.T. Bright ideas for chemical biology. ACS Chem. Biol. 3, 142–155 (2008).
Lavis, L.D. & Raines, R.T. Bright building blocks for chemical biology. ACS Chem. Biol. 9, 855–866 (2014).
Xue, L., Karpenko, I.A., Hiblot, J. & Johnsson, K. Imaging and manipulating proteins in live cells through covalent labeling. Nat. Chem. Biol. 11, 917–923 (2015).
Liu, Z., Lavis, L.D. & Betzig, E. Imaging live-cell dynamics and structure at the single-molecule level. Mol. Cell 58, 644–659 (2015).
Ceresole, M. Verfahren zur Darstellung von Farbstoffen aus der Gruppe des Meta-amidophenolphtaleïns. German Patent 44002 (1887).
Beija, M., Afonso, C.A.M. & Martinho, J.M.G. Synthesis and applications of Rhodamine derivatives as fluorescent probes. Chem. Soc. Rev. 38, 2410–2433 (2009).
Panchuk-Voloshina, N. et al. Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J. Histochem. Cytochem. 47, 1179–1188 (1999).
Arden-Jacob, J., Frantzeskos, J., Kemnitzer, N.U., Zilles, A. & Drexhage, K.H. New fluorescent markers for the red region. Spectrochim. Acta A Mol. Biomol. Spectrosc. 57, 2271–2283 (2001).
Liu, J.X. et al. Rational design and synthesis of a novel class of highly fluorescent rhodamine dyes that have strong absorption at long wavelengths. Tetrahedr. Lett. 44, 4355–4359 (2003).
Koide, Y., Urano, Y., Hanaoka, K., Terai, T. & Nagano, T. Evolution of group 14 rhodamines as platforms for near-infrared fluorescence probes utilizing photoinduced electron transfer. ACS Chem. Biol. 6, 600–608 (2011).
Grimm, J.B. et al. Carbofluoresceins and carborhodamines as scaffolds for high-contrast fluorogenic probes. ACS Chem. Biol. 8, 1303–1310 (2013).
Lukinavicius, G. et al. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nat. Chem. 5, 132–139 (2013).
Grimm, J.B. et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods 12, 244–250, 3, 250 (2015).
Lukinavicius, G. et al. Fluorogenic probes for multicolor imaging in living cells. J. Am. Chem. Soc. 138, 9365–9368 (2016).
Lavis, L.D., Chao, T.-Y. & Raines, R.T. Fluorogenic label for biomolecular imaging. ACS Chem. Biol. 1, 252–260 (2006).
Watkins, R.W., Lavis, L.D., Kung, V.M., Los, G.V. & Raines, R.T. Fluorogenic affinity label for the facile, rapid imaging of proteins in live cells. Org. Biomol. Chem. 7, 3969–3975 (2009).
Wysocki, L.M. et al. Facile and general synthesis of photoactivatable xanthene dyes. Angew. Chem. Int. Ed. Engl. 50, 11206–11209 (2011).
Lukinavicius, G. et al. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat. Methods 11, 731–733 (2014).
Butkevich, A.N. et al. Fluorescent rhodamines and fluorogenic carbopyronines for super-resolution STED microscopy in living cells. Angew. Chem. Int. Ed. Engl. 55, 3290–3294 (2016).
Grimm, J.B. et al. Synthesis of a far-red photoactivatable silicon-containing rhodamine for super-resolution microscopy. Angew. Chem. Int. Ed. Engl. 55, 1723–1727 (2016).
Grimm, J.B. & Lavis, L.D. Synthesis of rhodamines from fluoresceins using Pd-catalyzed C-N cross-coupling. Org. Lett. 13, 6354–6357 (2011).
Liu, Z. et al. 3D imaging of Sox2 enhancer clusters in embryonic stem cells. eLife 3, e04236 (2014).
Knight, S.C. et al. Dynamics of CRISPR-Cas9 genome interrogation in living cells. Science 350, 823–826 (2015).
Swinstead, E.E. et al. Steroid receptors reprogram FoxA1 occupancy through dynamic chromatin transitions. Cell 165, 593–605 (2016).
Bisson-Filho, A.W. et al. Treadmilling by FtsZ filaments drives peptidoglycan synthesis and bacterial cell division. Science 355, 739–743 (2017).
Grimm, J.B. et al. Bright photoactivatable fluorophores for single-molecule imaging. Nat. Methods 13, 985–988 (2016).
Whitaker, J.E. et al. Fluorescent rhodol derivatives: versatile, photostable labels and tracers. Anal. Biochem. 207, 267–279 (1992).
Mitronova, G.Y. et al. New fluorinated rhodamines for optical microscopy and nanoscopy. Chemistry 16, 4477–4488 (2010).
Asanuma, D. et al. Acidic-pH-activatable fluorescence probes for visualizing exocytosis dynamics. Angew. Chem. Int. Ed. Engl. 53, 6085–6089 (2014).
Hansch, C., Leo, A. & Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991).
Hinckley, D.A. & Seybold, P.G. A spectroscopic/thermodynamic study of the rhodamine B lactone–zwitterion equilibrium. Spectrochim. Acta A Mol. Biomol. Spectrosc. 44, 1053–1059 (1988).
Los, G.V. et al. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol. 3, 373–382 (2008).
Legant, W.R. et al. High-density three-dimensional localization microscopy across large volumes. Nat. Methods 13, 359–365 (2016).
Ohyama, T. et al. A multilevel multimodal circuit enhances action selection in Drosophila. Nature 520, 633–639 (2015).
Lemon, W.C. et al. Whole-central nervous system functional imaging in larval Drosophila. Nat. Commun. 6, 7924 (2015).
Chen, T.W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Zhou, X., Lai, R., Beck, J.R., Li, H. & Stains, C.I. Nebraska Red: a phosphinate-based near-infrared fluorophore scaffold for chemical biology applications. Chem. Commun. (Camb.) 52, 12290–12293 (2016).
Bruchez, M.P. Dark dyes-bright complexes: fluorogenic protein labeling. Curr. Opin. Chem. Biol. 27, 18–23 (2015).
Altman, R.B. et al. Cyanine fluorophore derivatives with enhanced photostability. Nat. Methods 9, 68–71 (2011).
Palonpon, A.F., Sodeoka, M. & Fujita, K. Molecular imaging of live cells by Raman microscopy. Curr. Opin. Chem. Biol. 17, 708–715 (2013).
Critchfield, F.E., Gibson, J.A. Jr. & Hall, J.L. Dielectric constant for the dioxane-water system from 20 to 35°. J. Am. Chem. Soc. 75, 1991–1992 (1953).
Suzuki, K. et al. Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector. Phys. Chem. Chem. Phys. 11, 9850–9860 (2009).
Frisch, M.J. et al. Gaussian 09, revision D.01. (Gaussian, Wallingford, Connecticut, USA, 2009).
Dreuw, A., Weisman, J.L. & Head-Gordon, M. Long-range charge-transfer excited states in time-dependent density functional theory require non-local exchange. J. Chem. Phys. 119, 2943–2946 (2003).
Jacquemin, D. et al. Assessment of the efficiency of long-range corrected functionals for some properties of large compounds. J. Chem. Phys. 126, 144105 (2007).
Guthmuller, J. & Champagne, B. Resonance Raman scattering of rhodamine 6G as calculated by time-dependent density functional theory: vibronic and solvent effects. J. Phys. Chem. A 112, 3215–3223 (2008).
Setiawan, D., Kazaryan, A., Martoprawiro, M.A. & Filatov, M. A first principles study of fluorescence quenching in rhodamine B dimers: how can quenching occur in dimeric species? Phys. Chem. Chem. Phys. 12, 11238–11244 (2010).
Mütze, J. et al. Excitation spectra and brightness optimization of two-photon excited probes. Biophys. J. 102, 934–944 (2012).
Akerboom, J. et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 32, 13819–13840 (2012).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63 (1983).
Kohl, J. et al. Ultrafast tissue staining with chemical tags. Proc. Natl. Acad. Sci. USA 111, E3805–E3814 (2014).
Ji, N., Milkie, D.E. & Betzig, E. Adaptive optics via pupil segmentation for high-resolution imaging in biological tissues. Nat. Methods 7, 141–147 (2010).
Sun, W., Tan, Z., Mensh, B.D. & Ji, N. Thalamus provides layer 4 of primary visual cortex with orientation- and direction-tuned inputs. Nat. Neurosci. 19, 308–315 (2016).
Acknowledgements
We thank A. Berro and E. Schreiter (Janelia) for purified HaloTag protein, and H. Choi (Janelia) for the Sec61β-HaloTag plasmid, contributive discussions and critical reading of the manuscript. This work was supported by the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
L.D.L. and J.B.G. conceived the project. J.B.G. contributed organic synthesis and one-photon spectroscopy measurements. A.K.M. contributed organic synthesis and computational chemistry experiments. Y.L., R.L. and N.J. contributed mouse imaging experiments. T.A.B. contributed cultured cell imaging experiments. W.C.L. and P.J.K. contributed larval explant imaging experiments. R.P. and J.J.M. contributed two-photon spectroscopy measurements. L.D.L. contributed one-photon spectroscopy measurements and wrote the manuscript with input from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare competing interests: J.B.G. and L.D.L. have filed patent applications whose value may be affected by this publication.
Integrated supplementary information
Supplementary Figure 1 Spectral data for fluorophores 1–12, 16, 21, and 25.
Normalized absorbance (abs), fluorescence excitation (flex), and fluorescence emission (flem) spectra for rhodamines (1, 5–12), rhodols (4, 16) carborhodamines (2, 21), and Si‑rhodamines (3, 25) in 10 mM HEPES, pH 7.3; the flex spectra are delineated by a dashed line. Note: the normalized absorbance spectra of fluorophores 3, 21, and 25 exhibit higher noise due to low visible absorption in aqueous buffer.
Supplementary Figure 2 Labeling cells with Janelia Fluor dyes.
(a) Chemical structure of JF525–SNAP-tag (15). (b) Image of COS7 cells expressing SNAP-tag–histone H2B and stained with ligand 15. (c) HaloTag and SNAP-tag ligands have no effect on COS7 cell viability at concentrations used for labeling: HaloTag ligands 13, 14, 17, 23, 26, 27 were incubated with cells for 1 h; SNAP-tag ligands 15, 20, 24, 29 were incubated for 3 h; error bars show ± s.d.; n = 3. (d) Chemical structures of known 488 nm-excited HaloTag ligands 18 and 19. (e) Plot of average cellular fluorescence vs. incubation time for live cells loaded with ligands 17–19. (f) Chemical structure of JF503–SNAP-tag ligand (20). (g) Image of COS7 cells expressing SNAP-tag–histone H2B and stained with ligand 20. (h) Structure of JF585–SNAP-tag ligand (24). (i) Image of COS7 cells expressing histone H2B–SNAP-tag and stained with ligand 24. (j) Multicolor image of U2OS cells expressing Sec61β–HaloTag fusion (stable) and TOMM20–SNAP-tag (transient) labeled with JF503–SNAP-tag ligand 20 (mitochondria, green), JF585-HaloTag ligand 23 (ER, orange), and JF646–Hoechst33 (nucleus, red). (k) Chemical structure of SiTMR–HaloTag ligand 28. (l) Images of COS7 cells expressing HaloTag–histone H2B fusion and labeled with 250 nM of HaloTag ligand 28 for 1 h and imaged directly without washing. The number indicates mean signal (nuclear) to background (cytosol) ratio (S/B) in three fields of view (n = 152 areas). This image was taken with identical microscope settings to those used with ligands 26 and 27 (Fig. 2m,n). (m) Chemical structure of JF635–SNAP-tag ligand (29). (n) Image of COS7 cells expressing SNAP-tag–histone H2B and stained with ligand 29. (o) Multicolor image of U2OS cells expressing Sec61β–HaloTag fusion (stable) and histone H2B–SNAP-tag (transient) labeled with JF525–SNAP-tag ligand 15 (nucleus, yellow) and JF635–HaloTag ligand 27 (ER, red). Scale bars for all images: 15 μm.
Supplementary Figure 3 In vivo imaging using the Janelia Fluor dyes
(a–b) Comparison of Basin cell and pan-neronal labeling in tissue. (a) SiMView light-sheet microscopy image of the ventral nerve cord region of Drosophila larval explant expressing HaloTag protein in Basin neurons and stained with JF635–HaloTag ligand (27; same imaging data set as Fig. 3a). Lower panel shows image of the anteroposterior (AP) cross-section of the indicated volume. (b) SiMView light-sheet microscopy image of ventral nerve cord region of Drosophila larval explant expressing GCaMP6s protein pan-neuronally (Gal4/UAS system; 57C10-Gal4 driver line). Lower panel shows image of the AP cross-section of the indicated volume. Scale bars for a and b: 50 μm. (c) SiMView light-sheet microscopy image (same as Fig. 3a) with inset showing a single imaging slice from the 3D projection through neuronal cell bodies. (d) Representative images from the labeling time course for JF585–HaloTag ligand (23) in vivo. Bright field image showing cranial window and epi-fluorescence images of green (GCaMP6s; t = 0) and red (JF585, t = 0 and 6 h. Scale bar: 0.5 mm. (e) Plot of GCaMP6s green fluorescence vs. JF585 red fluorescence for 2-photon imaging experiments. Found: Pearson linear correlation coefficient (ρ) = 0.768; n = 106 regions of interest (ROIs).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and Supplementary Note 1
Life Sciences Reporting Summary
Life Sciences Reporting Summary
In vivo labelling of layer 5 cortical neurons using JF585–HaloTag ligand
Layer 5 neurons expressing GCaMP6s and HaloTag were labeled with JF585–HaloTag ligand (23) through intraperitoneal (IP) injection and imaged with two-photon fluorescence microscopy. JF585 was excited at 1100 nm and the stack (307 μm × 307 μm × 530 μm) was acquired from 50 to 580 μm below dura mater at 2 μm step in Z. 3D movie was made by the ImageJ 3D view plugin (unit is in μm).
Rights and permissions
About this article
Cite this article
Grimm, J., Muthusamy, A., Liang, Y. et al. A general method to fine-tune fluorophores for live-cell and in vivo imaging. Nat Methods 14, 987–994 (2017). https://doi.org/10.1038/nmeth.4403
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.4403
This article is cited by
-
A rapid inducible RNA decay system reveals fast mRNA decay in P-bodies
Nature Communications (2024)
-
A general strategy to develop fluorogenic polymethine dyes for bioimaging
Nature Chemistry (2024)
-
Motion of VAPB molecules reveals ER–mitochondria contact site subdomains
Nature (2024)
-
Combined SPT and FCS methods reveal a mechanism of RNAP II oversampling in cell nuclei
Scientific Reports (2023)
-
A general highly efficient synthesis of biocompatible rhodamine dyes and probes for live-cell multicolor nanoscopy
Nature Communications (2023)