Abstract
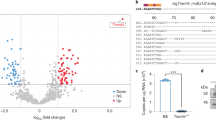
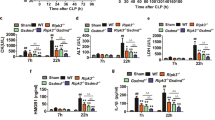
The function of the C5a receptors, C5ar (encoded by C5ar) and C5l2 (encoded by Gpr77), especially of C5l2, which was originally termed a 'default receptor', remains a controversial topic. Here we investigated the role of each receptor in the setting of cecal ligation and puncture–induced sepsis by using antibody-induced blockade of C5a receptors and knockout mice. In 'mid-grade' sepsis (30–40% survival), blockade or absence of either C5ar or C5l2 greatly improved survival and attenuated the buildup of proinflammatory mediators in plasma. In vivo appearance or in vitro release of high mobility group box 1 protein (HMGB1) required C5l2 but not C5ar. In 'high-grade' sepsis (100% lethality), the only protective condition was the combined blockade of C5l2 and C5ar. These data suggest that C5ar and C5l2 contribute synergistically to the harmful consequences in sepsis and that C5l2 is required for the release of HMGB1. Thus, contrary to earlier speculation, C5l2 is a functional receptor rather than merely a default receptor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Czermak, B.J. et al. Protective effects of C5a blockade in sepsis. Nat. Med. 5, 788–792 (1999).
Gerard, N.P. & Gerard, C. The chemotactic receptor for human C5a anaphylatoxin. Nature 349, 614–617 (1991).
Gerard, C. & Gerard, N.P. C5A anaphylatoxin and its seven transmembrane-segment receptor. Annu. Rev. Immunol. 12, 775–808 (1994).
Cain, S.A. & Monk, P.N. The orphan receptor C5L2 has high affinity binding sites for complement fragments C5a and C5a des-Arg74. J. Biol. Chem. 277, 7165–7169 (2002).
Johswich, K. et al. Ligand specificity of the anaphylatoxin C5L2 receptor and its regulation on myeloid and epithelial cell lines. J. Biol. Chem. 281, 39088–39095 (2006).
Okinaga, S. et al. C5L2, a nonsignaling C5A binding protein. Biochemistry 42, 9406–9415 (2003).
Kalant, D. et al. The chemoattractant receptor–like protein C5L2 binds the C3a des-Arg77/acylation-stimulating protein. J. Biol. Chem. 278, 11123–11129 (2003).
Kalant, D. et al. C5L2 is a functional receptor for acylation-stimulating protein. J. Biol. Chem. 280, 23936–23944 (2005).
Kildsgaard, J. et al. Cutting edge: targeted disruption of the C3a receptor gene demonstrates a novel protective anti-inflammatory role for C3a in endotoxin-shock. J. Immunol. 165, 5406–5409 (2000).
Takabayashi, T. et al. A new biologic role for C3a and C3a desArg: regulation of TNF-alpha and IL-1 beta synthesis. J. Immunol. 156, 3455–3460 (1996).
Gerard, N.P. et al. An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. J. Biol. Chem. 280, 39677–39680 (2005).
Johswich, K. & Klos, A. C5L2—an anti-inflammatory molecule or a receptor for acylation stimulating protein (C3a-desArg)? Adv. Exp. Med. Biol. 598, 159–180 (2007).
Chen, N.J. et al. C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature 446, 203–207 (2007).
Ohno, M. et al. A putative chemoattractant receptor, C5L2, is expressed in granulocyte and immature dendritic cells, but not in mature dendritic cells. Mol. Immunol. 37, 407–412 (2000).
Huber-Lang, M. et al. Changes in the novel orphan, C5a receptor (C5L2), during experimental sepsis and sepsis in humans. J. Immunol. 174, 1104–1110 (2005).
Hopken, U.E., Lu, B., Gerard, N.P. & Gerard, C. Impaired inflammatory responses in the reverse arthus reaction through genetic deletion of the C5a receptor. J. Exp. Med. 186, 749–756 (1997).
Otto, M. et al. C5a mutants are potent antagonists of the C5a receptor (CD88) and of C5L2: position 69 is the locus that determines agonism or antagonism. J. Biol. Chem. 279, 142–151 (2004).
Gao, H. et al. Evidence for a functional role of the second C5a receptor C5L2. FASEB J. 19, 1003–1005 (2005).
Lee, H. et al. Human C5aR knock-in mice facilitate the production and assessment of anti-inflammatory monoclonal antibodies. Nat. Biotechnol. 24, 1279–1284 (2006).
Czermak, B.J. et al. In vitro and in vivo dependency of chemokine generation on C5a and TNF-α. J. Immunol. 162, 2321–2325 (1999).
Gavrilyuk, V. et al. Identification of complement 5a-like receptor (C5L2) from astrocytes: characterization of anti-inflammatory properties. J. Neurochem. 92, 1140–1149 (2005).
Wang, H. et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 (1999).
Muller, S. et al. New EMBO members' review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 20, 4337–4340 (2001).
Lotze, M.T. & Tracey, K.J. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 5, 331–342 (2005).
Suda, K. et al. Anti–high-mobility group box chromosomal protein 1 antibodies improve survival of rats with sepsis. World J. Surg. 30, 1755–1762 (2006).
Yang, H. et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA 101, 296–301 (2004).
Qin, S. et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J. Exp. Med. 203, 1637–1642 (2006).
Hawlisch, H. et al. C5a negatively regulates Toll-like receptor 4–induced immune responses. Immunity 22, 415–426 (2005).
Zhang, X. et al. Regulation of Toll-like receptor–mediated inflammatory response by complement in vivo. Blood 110, 228–236 (2007).
Koleva, M. et al. Induction of anaphylatoxin C5a receptors in rat hepatocytes by lipopolysaccharide in vivo: mediation by interleukin-6 from Kupffer cells. Gastroenterology 122, 697–708 (2002).
Kohl, J. The role of complement in danger sensing and transmission. Immunol. Res. 34, 157–176 (2006).
Hawlisch, H. & Kohl, J. Complement and Toll-like receptors: key regulators of adaptive immune responses. Mol. Immunol. 43, 13–21 (2006).
Kim, J.Y. et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L958–L965 (2005).
Wang, H. et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 10, 1216–1221 (2004).
Bonaldi, T. et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 22, 5551–5560 (2003).
Godau, J. et al. C5a initiates the inflammatory cascade in immune complex peritonitis. J. Immunol. 173, 3437–3445 (2004).
Baelder, R. et al. Pharmacological targeting of anaphylatoxin receptors during the effector phase of allergic asthma suppresses airway hyperresponsiveness and airway inflammation. J. Immunol. 174, 783–789 (2005).
Yamada, S., Inoue, K., Yakabe, K., Imaizumi, H. & Maruyama, I. High mobility group protein 1 (HMGB1) quantified by ELISA with a monoclonal antibody that does not cross-react with HMGB2. Clin. Chem. 49, 1535–1537 (2003).
Acknowledgements
This work was supported by US National Institutes of Health grants GM-29507, GM-61656 and HL-31963 (to P.A.W.), AI-057839 (to J.K.) and HL-69511 (to C.G.) and Deutsche Forschungsgemeinschaft grants HU823/2-2 and HU823/2-3 (to M.H.-L.). We thank B. Schumann and S. Scott for secretarial assistance in preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
D.R. conducted all experiments and wrote the manuscript. M.A.F., B.A.N., D.E.D. and M.H.-L. contributed to the in vivo studies. C.R.M., F.S.Z. and N.P.G. made contributions to some of the in vitro experiments. K.C., J.K. and C.G. provided knockout mice and receptor antagonists. J.V.S. and P.A.W. supervised the project and edited the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Fig. 1 and Supplementary Table 1 (PDF 1419 kb)
Rights and permissions
About this article
Cite this article
Rittirsch, D., Flierl, M., Nadeau, B. et al. Functional roles for C5a receptors in sepsis. Nat Med 14, 551–557 (2008). https://doi.org/10.1038/nm1753
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1753
This article is cited by
-
Vitamin D receptor (VDR) on the cell membrane of mouse macrophages participates in the formation of lipopolysaccharide tolerance: mVDR is related to the effect of artesunate to reverse LPS tolerance
Cell Communication and Signaling (2023)
-
Mechanism of activation and biased signaling in complement receptor C5aR1
Cell Research (2023)
-
The Key Drivers of Brain Injury by Systemic Inflammatory Responses after Sepsis: Microglia and Neuroinflammation
Molecular Neurobiology (2023)
-
3′mRNA sequencing reveals pro-regenerative properties of c5ar1 during resolution of murine acetaminophen-induced liver injury
npj Regenerative Medicine (2022)
-
Role of the C5a-C5a receptor axis in the inflammatory responses of the lungs after experimental polytrauma and hemorrhagic shock
Scientific Reports (2021)