Abstract
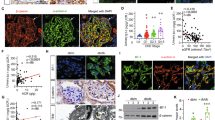
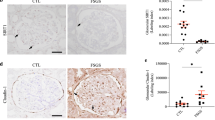
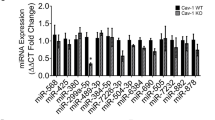
Focal segmental glomerulosclerosis (FSGS) is a frequent and severe glomerular disease characterized by destabilization of podocyte foot processes. We report that transgenic expression of the microRNA miR-193a in mice rapidly induces FSGS with extensive podocyte foot process effacement. Mechanistically, miR-193a inhibits the expression of the Wilms' tumor protein (WT1), a transcription factor and master regulator of podocyte differentiation and homeostasis. Decreased expression levels of WT1 lead to downregulation of its target genes PODXL (podocalyxin) and NPHS1 (nephrin), as well as several other genes crucial for the architecture of podocytes, initiating a catastrophic collapse of the entire podocyte-stabilizing system. We found upregulation of miR-193a in isolated glomeruli from individuals with FSGS compared to normal kidneys or individuals with other glomerular diseases. Thus, upregulation of miR-193a provides a new pathogenic mechanism for FSGS and is a potential therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
D'Agati, V.D., Kaskel, F.J. & Falk, R.J. Focal segmental glomerulosclerosis. N. Engl. J. Med. 365, 2398–2411 (2011).
Wiggins, R.C. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 71, 1205–1214 (2007).
Inui, M., Martello, G. & Piccolo, S. MicroRNA control of signal transduction. Nat. Rev. Mol. Cell Biol. 11, 252–263 (2010).
Kato, M., Park, J.T. & Natarajan, R. MicroRNA and the glomerulus. Exp. Cell Res. 318, 993–1000 (2012).
Bennett, M.R. et al. Laser capture microdissection-microarray analysis of focal segmental glomerulosclerosis glomeruli. Nephron Exp. Nephrol. 107, e30–e40 (2007).
Hodgin, J.B. et al. A molecular profile of focal segmental glomerulosclerosis from formalin-fixed, paraffin-embedded tissue. Am. J. Pathol. 177, 1674–1686 (2010).
Shi, S. et al. Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. J. Am. Soc. Nephrol. 19, 2159–2169 (2008).
Morrison, A.A., Viney, R.L., Saleem, M.A. & Ladomery, M.R. New insights into the function of the Wilm tumor suppressor gene WT1 in podocytes. Am. J. Physiol. Renal Physiol. 295, F12–F17 (2008).
Seibler, J. et al. Reversible gene knockdown in mice using a tight, inducible shRNA expression system. Nucleic Acids Res. 35, e54 (2007).
Takemoto, M. et al. A new method for large scale isolation of kidney glomeruli from mice. Am. J. Pathol. 161, 799–805 (2002).
Creighton, C.J., Nagaraja, A.K., Hanash, S.M., Matzuk, M.M. & Gunaratne, P.H. A bioinformatics tool for linking gene expression profiling results with public databases of microRNA target predictions. RNA 14, 2290–2296 (2008).
John, B., et al. Human MicroRNA targets. PLoS Biol. 2, e363 (2004).
Ohtaka, A., Ootaka, T., Sato, H. & Ito, S. Phenotypic change of glomerular podocytes in primary focal segmental glomerulosclerosis: developmental paradigm? Nephrol. Dial. Transplant. 17 (suppl. 9), 11–15 (2002).
Chau, Y.Y. et al. Acute multiple organ failure in adult mice deleted for the developmental regulator WT1. PLoS Genet. 7, e1002404 (2011).
Palmer, R.E. et al. WT1 regulates the expression of the major glomerular podocyte membrane protein Podocalyxin. Curr. Biol. 11, 1805–1809 (2001).
Wagner, N., Wagner, K.D., Xing, Y., Scholz, H. & Schedl, A. The major podocyte protein nephrin is transcriptionally activated by the Wilms' tumor suppressor WT1. J. Am. Soc. Nephrol. 15, 3044–3051 (2004).
Ratelade, J. et al. A murine model of Denys-Drash syndrome reveals novel transcriptional targets of WT1 in podocytes. Hum. Mol. Genet. 19, 1–15 (2010).
Schumacher, V.A. et al. WT1-dependent sulfatase expression maintains the normal glomerular filtration barrier. J. Am. Soc. Nephrol. 22, 1286–1296 (2011).
Kreidberg, J.A. et al. WT-1 is required for early kidney development. Cell 74, 679–691 (1993).
Shigehara, T. et al. Inducible podocyte-specific gene expression in transgenic mice. J. Am. Soc. Nephrol. 14, 1998–2003 (2003).
Schönig, K., Schwenk, F., Rajewsky, K. & Bujard, H. Stringent doxycycline dependent control of CRE recombinase in vivo. Nucleic Acids Res. 30, e134 (2002).
Venkatachalam, M.A., Cotran, R.S. & Karnovsky, M.J. An ultrastructural study of glomerular permeability in aminonucleoside nephrosis using catalase as a tracer protein. J. Exp. Med. 132, 1168–1180 (1970).
Kaplan, B.S., Renaud, L. & Drummond, K.N. Effects of aminonucleoside, daunomycin, and adriamycin on carbon oxidation by glomeruli. Lab Invest. 34, 174–178 (1976).
Binder, C.J., Weiher, H., Exner, M. & Kerjaschki, D. Glomerular overproduction of oxygen radicals in Mpv17 gene-inactivated mice causes podocyte foot process flattening and proteinuria: a model of steroid-resistant nephrosis sensitive to radical scavenger therapy. Am. J. Pathol. 154, 1067–1075 (1999).
Wei, C. et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat. Med. 17, 952–960 (2011).
Farquhar, M.G., Saito, A., Kerjaschki, D. & Orlando, R.A. The Heymann nephritis antigenic complex: megalin (gp330) and RAP. J. Am. Soc. Nephrol. 6, 35–47 (1995).
Seiler, M.W., Venkatachalam, M.A. & Cotran, R.S. Glomerular epithelium: structural alterations induced by polycations. Science 189, 390–393 (1975).
Kashtan, C., Fish, A.J., Kleppel, M., Yoshioka, K. & Michael, A.F. Nephritogenic antigen determinants in epidermal and renal basement membranes of kindreds with Alport-type familial nephritis. J. Clin. Invest. 78, 1035–1044 (1986).
Juhila, J. et al. Inducible nephrin transgene expression in podocytes rescues nephrin-deficient mice from perinatal death. Am. J. Pathol. 176, 51–63 (2010).
Barisoni, L., Bruggeman, L.A., Mundel, P., D'Agati, V.D. & Klotman, P.E. HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int. 58, 173–181 (2000).
Alpers, C.E. & Hudkins, K.L. Mouse models of diabetic nephropathy. Curr. Opin. Nephrol. Hypertens. 20, 278–284 (2011).
Obad, S. et al. Silencing of microRNA families by seed-targeting tiny LNAs. Nat. Genet. 43, 371–378 (2011).
Zhdanova, O. et al. The inducible deletion of Drosha and microRNAs in mature podocytes results in a collapsing glomerulopathy. Kidney Int. 80, 719–730 (2011).
Guo, J.K. et al. WT1 is a key regulator of podocyte function: reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Hum. Mol. Genet. 11, 651–659 (2002).
Ross, M.D. et al. Math6 expression during kidney development and altered expression in a mouse model of glomerulosclerosis. Dev. Dyn. 235, 3102–3109 (2006).
Kerjaschki, D., Sharkey, D.J. & Farquhar, M.G. Identification and characterization of podocalyxin—the major sialoprotein of the renal glomerulus. J. Cell Biol. 98, 1591–1596 (1984).
Takeda, T., McQuistan, T., Orlando, R.A. & Farquhar, M.G. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J. Clin. Invest. 108, 289–301 (2001).
Doyonnas, R. et al. Anuria, omphalocele and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J. Exp. Med. 194, 13–27 (2001).
Huber, T.B. & Benzing, T. The slit diaphragm: a signalling platform to regulate podocyte function. Curr. Opin. Nephrol. Hypertens. 14, 211–216 (2005).
Lenkkeri, U. et al. Structure of the gene for congenital nephrotic syndrome of the Finnish type (NPHS1) and characterization of mutations. Am. J. Hum. Genet. 64, 51–61 (1999).
Rantanen, M. et al. Nephrin TRAP mice lack slit diaphragms and show fibrotic glomeruli and cystic tubular lesions. J. Am. Soc. Nephrol. 13, 1586–1594 (2002).
Greka, A. & Mundel, P. Cell biology and pathology of podocytes. Annu. Rev. Physiol. 74, 299–323 (2012).
Welsh, G.I. & Saleem, M.A. The podocyte cytoskeleton—key to a functioning glomerulus in health and disease. Nat. Rev. Nephrol. 8, 14–21 (2012).
Giannico, G., Yang, H., Neilson, E.G. & Fogo, A.B. Dystroglycan in the diagnosis of FSGS. Clin. J. Am. Soc. Nephrol. 4, 1747–1753 (2009).
Seibler, J. et al. Single copy shRNA configuration for ubiquitous gene knockdown in mice. Nucleic Acids Res. 33, e67 (2005).
Wang, S. et al. Recipient Toll-like receptors contribute to chronic graft dysfunction by both MyD88- and TRIF-dependent signaling. Dis. Model Mech. 3, 92–103 (2010).
Mundel, P., Reiser, J. & Kriz, W. Induction of differentiation in cultured rat and human podocytes. J. Am. Soc. Nephrol. 8, 697–705 (1997).
Saleem, M.A. et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 13, 630–638 (2002).
Grant, G.R. et al. Comparative analysis of RNA-Seq alignment algorithms and the RNA-Seq unified mapper (RUM). Bioinformatics 27, 2518–2528 (2011).
Kerjaschki, D. et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat. Med. 12, 230–234 (2006).
Michael, A.F., Blau, E. & Vernier, R.L. Glomerular polyanion: alteration in aminonucleoside nephrosis. Lab. Invest. 23, 649–657 (1970).
Acknowledgements
This study was supported in part by E-Rare JTC 2011, European Research Projects on Rare Diseases, Project # I 923-B13 to D.K. and M.J.M. and a grant from the Deutsche Forschungsgemeinschaft (EN 280/8-1) to C.E. We thank A. Fogo (Vanderbilt University), J. Reiser (Rush University Medical Center) and C. Alpers (University of Washington) for sending us paraffin blocks of kidneys from animal models of proteinuria. Immortalized mouse podocytes were a kind gift of P. Mundel (MGH Boston). Immortalized human podocytes were a gift of L. Schaefer, University of Frankfurt. We thank K. Priessner, U. Rothermel, D. Kruspe, S. Krieger, H. Schachner, G. Asfour, I. Raab, M. Volz and A. Jäger for expert technical assistance and A. Rees for critical reading of the manuscript. Cell sorting was performed by A. Spittler and G. Hofbauer from the Core Facility Cell Sorting, Medical University of Vienna.
Author information
Authors and Affiliations
Contributions
D.K., C.A.G. and J.M. conceived the project. C.A.G. and D.K. designed the experiments and wrote the paper. C.A.G. and C.K. performed the experiments related to miR-193a. S.T. and M.B. performed the RNA-Seq analysis. C.K., G.A.B., R.K. and M.J.M. selected the human samples. C.E., H.-J.G., L.D. and R.S. generated and characterized the conditional WT1 knockout mouse. J.S. and M.R. generated the inducible miR-193a transgenic mouse. S.W. and H.-J.G. performed the kidney transplantation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Tables 2–4 (PDF 1046 kb)
Supplementary Table 1
Genes regulated in isolated mouse glomeruli upon miR-193a (XLS 9235 kb)
Rights and permissions
About this article
Cite this article
Gebeshuber, C., Kornauth, C., Dong, L. et al. Focal segmental glomerulosclerosis is induced by microRNA-193a and its downregulation of WT1. Nat Med 19, 481–487 (2013). https://doi.org/10.1038/nm.3142
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3142
This article is cited by
-
Extracellular vesicles in kidney development and pediatric kidney diseases
Pediatric Nephrology (2023)
-
MicroRNAs in kidney injury and disease
Nature Reviews Nephrology (2022)
-
Alteration in DNA-binding affinity of Wilms tumor 1 protein due to WT1 genetic variants associated with steroid - resistant nephrotic syndrome in children
Scientific Reports (2022)
-
Cytoplasmic WT1 in IgA nephropathy, an indicator of poor prognosis associated with mesangial/peri-mesangial C4d
International Urology and Nephrology (2022)
-
The recruitment mechanisms and potential therapeutic targets of podocytes from parietal epithelial cells
Journal of Translational Medicine (2021)