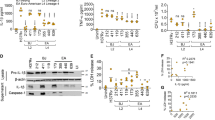
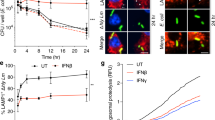
Abstract
Interleukin 1 (IL-1) is an important mediator of innate immunity but can also promote inflammatory tissue damage. During chronic infections such as tuberculosis, the beneficial antimicrobial role of IL-1 must be balanced with the need to prevent immunopathology. By exogenously controlling the replication of Mycobacterium tuberculosis in vivo, we obviated the requirement for antimicrobial immunity and discovered that both IL-1 production and infection-induced immunopathology were suppressed by lymphocyte-derived interferon-γ (IFN-γ). This effect was mediated by nitric oxide (NO), which we found specifically inhibited assembly of the NLRP3 inflammasome via thiol nitrosylation. Our data indicate that the NO produced as a result of adaptive immunity is indispensable in modulating the destructive innate inflammatory responses elicited during persistent infections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Lamkanfi, M. & Dixit, V.M. Inflammasomes: guardians of cytosolic sanctity. Immunol. Rev. 227, 95–105 (2009).
Vance, R.E., Isberg, R.R. & Portnoy, D.A. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe 6, 10–21 (2009).
Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Dinarello, C.A. Role of interleukin-1 in infectious diseases. Immunol. Rev. 127, 119–146 (1992).
Hise, A.G. et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5, 487–497 (2009).
Nathan, C. & Ding, A. Nonresolving inflammation. Cell 140, 871–882 (2010).
Pandey, A.K. et al. NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog. 5, e1000500 (2009).
Coulombe, F. et al. Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J. Exp. Med. 206, 1709–1716 (2009).
Kang, D.D., Lin, Y., Moreno, J.R., Randall, T.D. & Khader, S.A. Profiling early lung immune responses in the mouse model of tuberculosis. PLoS ONE 6, e16161 (2011).
Seiler, P. et al. Early granuloma formation after aerosol Mycobacterium tuberculosis infection is regulated by neutrophils via CXCR3-signaling chemokines. Eur. J. Immunol. 33, 2676–2686 (2003).
MacMicking, J.D. et al. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94, 5243–5248 (1997).
Capuano, S.V. III et al. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect. Immun. 71, 5831–5844 (2003).
North, R.J. & Jung, Y.J. Immunity to tuberculosis. Annu. Rev. Immunol. 22, 599–623 (2004).
Chackerian, A.A., Perera, T.V. & Behar, S.M. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infect. Immun. 69, 2666–2674 (2001).
Eum, S.Y. et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 137, 122–128 (2010).
Eruslanov, E.B. et al. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect. Immun. 73, 1744–1753 (2005).
Carlsson, F. et al. Host-detrimental role of Esx-1-mediated inflammasome activation in mycobacterial infection. PLoS Pathog. 6, e1000895 (2010).
Mogues, T., Goodrich, M.E. & Ryan, L. LaCourse, R. & North, R.J. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193, 271–280 (2001).
Nandi, B. & Behar, S.M. Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J. Exp. Med. 208, 2251–2262 (2011).
Minguela, A., Pastor, S., Mi, W., Richardson, J.A. & Ward, E.S. Feedback regulation of murine autoimmunity via dominant anti-inflammatory effects of interferon gamma. J. Immunol. 178, 134–144 (2007).
Honoré, N., Marchal, G. & Cole, S.T. Novel mutation in 16S rRNA associated with streptomycin dependence in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 39, 769–770 (1995).
Kashino, S.S., Ovendale, P., Izzo, A. & Campos-Neto, A. Unique model of dormant infection for tuberculosis vaccine development. Clin. Vaccine Immunol. 13, 1014–1021 (2006).
McElvania TeKippe, E. et al. Granuloma formation and host defense in chronic Mycobacterium tuberculosis infection requires PYCARD/ASC but not NLRP3 or caspase-1. PLoS ONE 5, e12320 (2010).
Mishra, B.B. et al. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell. Microbiol. 12, 1046–1063 (2010).
Mayer-Barber, K.D. et al. Innate and adaptive interferons suppress IL-1alpha and IL-1beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity 35, 1023–1034 (2011).
Mayer-Barber, K.D. et al. Caspase-1 independent IL-1β production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184, 3326–3330 (2010).
Keller, M., Ruegg, A., Werner, S. & Beer, H.D. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831 (2008).
Pelegrin, P. & Surprenant, A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J. Biol. Chem. 282, 2386–2394 (2007).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Franchi, L. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat. Immunol. 7, 576–582 (2006).
Koo, I.C. et al. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell. Microbiol. 10, 1866–1878 (2008).
Broz, P. et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J. Exp. Med. 207, 1745–1755 (2010).
Chan, J., Tanaka, K., Carroll, D., Flynn, J. & Bloom, B.R. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect. Immun. 63, 736–740 (1995).
Hobbs, A.J. & Ignarro, L.J. Nitric oxide-cyclic GMP signal transduction system. Methods Enzymol. 269, 134–148 (1996).
Fernandes-Alnemri, T. et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14, 1590–1604 (2007).
Stamler, J.S., Lamas, S. & Fang, F.C. Nitrosylation. the prototypic redox-based signaling mechanism. Cell 106, 675–683 (2001).
Forrester, M.T., Foster, M.W. & Stamler, J.S. Assessment and application of the biotin switch technique for examining protein S-nitrosylation under conditions of pharmacologically induced oxidative stress. J. Biol. Chem. 282, 13977–13983 (2007).
Jaffrey, S.R. & Snyder, S.H. The biotin switch method for the detection of S-nitrosylated proteins. Sci. STKE 2001, pl1 (2001).
Dimmeler, S., Haendeler, J., Nehls, M. & Zeiher, A.M. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1β-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J. Exp. Med. 185, 601–607 (1997).
Broz, P., von Moltke, J., Jones, J.W., Vance, R.E. & Monack, D.M. Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8, 471–483 (2010).
Rathinam, V.A., Vanaja, S.K. & Fitzgerald, K.A. Regulation of inflammasome signaling. Nat. Immunol. 13, 333–332 (2012).
Meissner, F., Molawi, K. & Zychlinsky, A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat. Immunol. 9, 866–872 (2008).
Hopkins, N., Gunning, Y., O'Croinin, D.F., Laffey, J.G. & McLoughlin, P. Anti-inflammatory effect of augmented nitric oxide production in chronic lung infection. J. Pathol. 209, 198–205 (2006).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Nandi, B. & Behar, S.M. Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J. Exp. Med. 208, 2251–2262 (2011).
Fremond, C.M. et al. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J. Immunol. 179, 1178–1189 (2007).
Tobin, D.M. et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell 140, 717–730 (2010).
Schön, T. et al. Arginine as an adjuvant to chemotherapy improves clinical outcome in active tuberculosis. Eur. Respir. J. 21, 483–488 (2003).
Martens, G.W. et al. Tuberculosis susceptibility of diabetic mice. Am. J. Respir. Cell Mol. Biol. 37, 518–524 (2007).
Roberson, S.M. & Walker, W.S. Immortalization of cloned mouse splenic macrophages with a retrovirus containing the v-raf/mil and v-myc oncogenes. Cell. Immunol. 116, 341–351 (1988).
Acknowledgements
We thank S. Swain (University of Massachusetts Medical School) for Ifngr1−/− mice; A. Campos-Neto (Forsyth Institute) for M. tuberculosis strain 18b; B. McCormick (University of Massachusetts Medical School) for Salmonella enterica serovar Typhimurium; E. Alnemri (Thomas Jefferson University) for rabbit polyclonal antibody to mouse AIM2; G. Nunez (University of Michigan) for rat monoclonal antibody to ASC; B. McCormick, K. Rock, R. Mishra, A.K. Pandey and J. Lee for scientific insight; and H. Ducharme for animal husbandry. Supported by the US National Institutes of Health (AI064282 to C.M.S.; HL064884 to H.K.; AI083713 to K.A.F.; and U54 AI057159 to V.A.K.R.) and the Howard Hughes Medical Institute (C.M.S.).
Author information
Authors and Affiliations
Contributions
B.B.M. and C.M.S. conceived of the project and designed the experiments; B.B.M. did most of the experiments; V.A.K.R. and G.W.M. did specific experiments; A.J.M. provided veterinary pathology expertise; B.B.M. and C.M.S. wrote the manuscript; H.K. and K.A.F. provided mutant mice, scientific insight and critical review of the manuscript; and C.M.S. oversaw the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 872 kb)
Rights and permissions
About this article
Cite this article
Mishra, B., Rathinam, V., Martens, G. et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome–dependent processing of IL-1β. Nat Immunol 14, 52–60 (2013). https://doi.org/10.1038/ni.2474
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2474
This article is cited by
-
Zingerone Alleviates Morphine Tolerance and Dependence in Mice by Reducing Oxidative Stress-Mediated NLRP3 Inflammasome Activation
Neurochemical Research (2024)
-
Therapeutic effect of Echinococcus granulosus cyst fluid on bacterial sepsis in mice
Parasites & Vectors (2023)
-
An innate granuloma eradicates an environmental pathogen using Gsdmd and Nos2
Nature Communications (2023)
-
Glycerol contributes to tuberculosis susceptibility in male mice with type 2 diabetes
Nature Communications (2023)
-
Helper T cell bias following tuberculosis chemotherapy identifies opportunities for therapeutic vaccination to prevent relapse
npj Vaccines (2023)