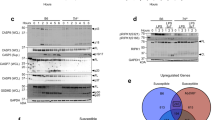
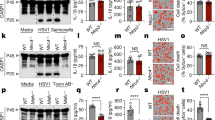
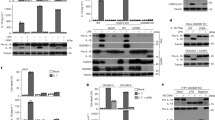
Abstract
Production of the proinflammatory cytokine interleukin 1β (IL-1β) by dendritic cells is crucial in host defense. Here we identify a previously unknown role for dectin-1 in the activation of a noncanonical caspase-8 inflammasome in response to fungi and mycobacteria. Dectin-1 induced both the production and maturation of IL-1β through signaling routes mediated by the kinase Syk. Whereas the CARD9–Bcl-10–MALT1 scaffold directed IL1B transcription, the recruitment of MALT1–caspase-8 and ASC into this scaffold was crucial for processing of pro-IL-1β by caspase-8. In contrast to activation of the canonical caspase-1 inflammasome, which requires additional activation of cytosolic receptors, activation of the noncanonical caspase-8 inflammasome was independent of pathogen internalization. Thus, dectin-1 acted as an extracellular sensor for pathogens that induced both IL-1β production and maturation through a noncanonical caspase-8-dependent inflammasome for protective immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
03 February 2012
In the version of this article initially published online, the first sentence of the third paragraph in the Discussion section was incorrect. It should begin "In contrast to the processing of pro-IL-1β via caspase-8...". The error has been corrected for the print, PDF and HTML versions of this article.
References
Romani, L. Cell mediated immunity to fungi: a reassessment. Med. Mycol. 46, 515–529 (2008).
Segal, B.H. Aspergillosis. N. Engl. J. Med. 360, 1870–1884 (2009).
van Beelen, A.J. et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 27, 660–669 (2007).
Weaver, C.T. & Hatton, R.D. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat. Rev. Immunol. 9, 883–889 (2009).
Louten, J., Boniface, K. & de Waal Malefyt, R. Development and function of TH17 cells in health and disease. J. Allergy Clin. Immunol. 123, 1004–1011 (2009).
Ouyang, W., Kolls, J.K. & Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28, 454–467 (2008).
Geijtenbeek, T.B.H. & Gringhuis, S.I. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 9, 465–479 (2009).
Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 (2007).
Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 11, 275–288 (2011).
Gross, O. et al. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 442, 651–656 (2006).
LeibundGut-Landmann, S. et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8, 630–638 (2007).
Gringhuis, S.I. et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-κB activation through Raf-1 and Syk. Nat. Immunol. 10, 203–213 (2009).
Ferwerda, B. et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 361, 1760–1767 (2009).
Rogers, N.C. et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22, 507–517 (2005).
Rawlings, D.J., Sommer, K. & Moreno-Garcia, M.E. The CARMA1 signalosome links the signalling machinery of adaptive and innate immunity in lymphocytes. Nat. Rev. Immunol. 6, 799–812 (2006).
Gringhuis, S.I. et al. Selective c-Rel activation via Malt1 controls anti-fungal TH-17 immunity by dectin-1 and dectin-2. PLoS Pathog. 7, e1001259 (2011).
Netea, M.G. et al. IL-1β processing in host defense: beyond the inflammasomes. PLoS Pathog. 6, e1000661 (2010).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Poeck, H. et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1β production. Nat. Immunol. 11, 63–69 (2010).
Schroder, K., Zhou, R. & Tschopp, J. The NLRP3 inflammasome: a sensor for metabolic danger? Science 327, 296–300 (2010).
Tschopp, J. & Schroder, K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10, 210–215 (2010).
Gross, O. et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459, 433–436 (2009).
Saïd-Sadier, N., Padilla, E., Langsley, G. & Ojcius, D.M. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS ONE 5, e10008 (2010).
Hise, A.G. et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5, 487–497 (2009).
Mariathasan, S. & Monack, D.M. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 7, 31–40 (2007).
Maelfait, J. et al. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1β maturation by caspase-8. J. Exp. Med. 205, 1967–1973 (2008).
Maelfait, J. & Beyaert, R. Non-apoptotic functions of caspase-8. Biochem. Pharmacol. 76, 1365–1373 (2008).
Oberst, A. & Green, D.R. It cuts both ways: reconciling the dual roles of caspase 8 in cell death and survival. Nat. Rev. Mol. Cell Biol. 12, 757–763 (2011).
Kawadler, H., Gantz, M.A., Riley, J.L. & Yang, X. The paracaspase MALT1 controls caspase-8 activation during lymphocyte proliferation. Mol. Cell 31, 415–421 (2008).
Thome, M. Multifunctional roles for MALT1 in T-cell activation. Nat. Rev. Immunol. 8, 495–500 (2008).
Rebeaud, F. et al. The proteolytic activity of the paracaspase MALT1 is key in T cell activation. Nat. Immunol. 9, 272–281 (2008).
Goddette, D.W. & Frieden, C. Actin polymerization. The mechanism of action of cytochalasin D. J. Biol. Chem. 261, 15974–15980 (1986).
Hohl, T.M. et al. Aspergillus fumigatus triggers inflammatory responses by stage-specific β-glucan display. PLoS Pathog. 1, e30 (2005).
Zenaro, E., Donini, M. & Dusi, S. Induction of Th1/Th17 immune response by Mycobacterium tuberculosis: role of dectin-1, mannose receptor, and DC-SIGN. J. Leukoc. Biol. 86, 1393–1401 (2009).
Rothfuchs, A.G. et al. Dectin-1 interaction with Mycobacterium tuberculosis leads to enhanced IL-12p40 production by splenic dendritic cells. J. Immunol. 179, 3463–3471 (2007).
van de Veerdonk, F.L. et al. Mycobacterium tuberculosis induces IL-17A responses through TLR4 and dectin-1 and is critically dependent on endogenous IL-1. J. Leukoc. Biol. 88, 227–232 (2010).
Masumoto, J. et al. ASC is an activating adaptor for NF-κB and caspase-8-dependent apoptosis. Biochem. Biophys. Res. Commun. 303, 69–73 (2003).
Faustin, B. et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell 25, 713–724 (2007).
Glocker, E.O. et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 361, 1727–1735 (2009).
Bellocchio, S. et al. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 172, 3059–3069 (2004).
Vonk, A. et al. Endogenous interleukin (IL)-1α and IL-1β are crucial for host defense against disseminated candidiasis. J. Infect. Dis. 193, 1419–1426 (2006).
Mencacci, A. et al. Interleukin 18 restores defective Th1 immunity to Candida albicans in caspase 1-deficient mice. Infect. Immun. 68, 5126–5131 (2000).
Fantuzzi, G. et al. Response to local inflammation of IL-1β-converting enzyme-deficient mice. J. Immunol. 158, 1818–1824 (1997).
Geahlen, R.L. & McLaughlin, J.L. Piceatannol (3,4,3′,5′-tetrahydroxy-trans-stilbene) is a naturally occurring protein-tyrosine kinase inhibitor. Biochem. Biophys. Res. Commun. 165, 241–245 (1989).
Nicholson, D.W. et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376, 37–43 (1995).
Garcia-Calvo, M. et al. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J. Biol. Chem. 273, 32608–32613 (1998).
Acknowledgements
We thank J. Belisle (Colorado State University) for M. tuberculosis (as part of HHSN266200400091C (“Tuberculosis Vaccine Testing and Research Materials”) from the National Institute of Allergy and Infectious Diseases of the US National Institutes of Health); B. Appelmelk (VU Medical Center, Amsterdam) for M. bovis BCG; and J. Spencer (Colorado State University) for M. leprae. Supported by the Netherlands Organisation for Scientific Research (Netherlands Genomic Initiative 40-41009-98-8057 to S.I.G.; Vici 918.10.619 to T.B.H.G.) and the AIDS Foundation (2007036 to M.v.d.V.).
Author information
Authors and Affiliations
Contributions
S.I.G. designed and supervised all aspects of this study, did and interpreted most experiments and prepared the manuscript; T.M.K. did fungi stimulations, enzyme-linked immunosorbent assay (ELISA) of cytokines, flow cytometry and assays of caspase-8 and internalization; B.A.W. did ELISA of cytokines; B.T. and T.B. prepared the fungal strains; M.v.d.V. did silencing experiments; and T.B.H.G. supervised all aspects of this study and prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Table 1 (PDF 1311 kb)
Rights and permissions
About this article
Cite this article
Gringhuis, S., Kaptein, T., Wevers, B. et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat Immunol 13, 246–254 (2012). https://doi.org/10.1038/ni.2222
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2222
This article is cited by
-
Involvement of inflammasomes in tumor microenvironment and tumor therapies
Journal of Hematology & Oncology (2023)
-
The NLR gene family: from discovery to present day
Nature Reviews Immunology (2023)
-
Gasdermins and pyroptosis in the kidney
Nature Reviews Nephrology (2023)
-
EV-A71 induced IL-1β production in THP-1 macrophages is dependent on NLRP3, RIG-I, and TLR3
Scientific Reports (2022)
-
DOCK2 regulates antifungal immunity by regulating RAC GTPase activity
Cellular & Molecular Immunology (2022)