Abstract
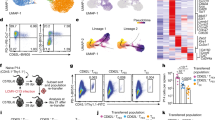
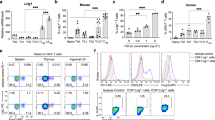
The mechanisms that regulate T cell quiescence are poorly understood. We report that the tumor suppressor Tsc1 established a quiescence program in naive T cells by controlling cell size, cell cycle entry and responses to stimulation of the T cell antigen receptor. Abrogation of quiescence predisposed Tsc1-deficient T cells to apoptosis that resulted in loss of conventional T cells and invariant natural killer T cells. Loss of Tsc1 function dampened in vivo immune responses to bacterial infection. Tsc1-deficient T cells had more activity of the serine-threonine kinase complex mTORC1 but less mTORC2 activity, and activation of mTORC1 was essential for the disruption of immune homeostasis. Therefore, Tsc1-dependent control of mTOR is crucial in actively maintaining the quiescence of naive T cells to facilitate adaptive immune function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Hedrick, S.M., Ch'en, I.L. & Alves, B.N. Intertwined pathways of programmed cell death in immunity. Immunol. Rev. 236, 41–53 (2010).
Surh, C.D. & Sprent, J. Homeostasis of naive and memory T cells. Immunity 29, 848–862 (2008).
Takada, K. & Jameson, S.C. Naive T cell homeostasis: from awareness of space to a sense of place. Nat. Rev. Immunol. 9, 823–832 (2009).
Glynne, R., Ghandour, G., Rayner, J., Mack, D.H. & Goodnow, C.C. B-lymphocyte quiescence, tolerance and activation as viewed by global gene expression profiling on microarrays. Immunol. Rev. 176, 216–246 (2000).
Teague, T.K. et al. Activation changes the spectrum but not the diversity of genes expressed by T cells. Proc. Natl. Acad. Sci. USA 96, 12691–12696 (1999).
Berger, M. et al. An Slfn2 mutation causes lymphoid and myeloid immunodeficiency due to loss of immune cell quiescence. Nat. Immunol. 11, 335–343 (2010).
Modiano, J.F., Johnson, L.D. & Bellgrau, D. Negative regulators in homeostasis of naive peripheral T cells. Immunol. Res. 41, 137–153 (2008).
Yusuf, I. & Fruman, D.A. Regulation of quiescence in lymphocytes. Trends Immunol. 24, 380–386 (2003).
Kuo, C.T., Veselits, M.L. & Leiden, J.M. LKLF: A transcriptional regulator of single-positive T cell quiescence and survival. Science 277, 1986–1990 (1997).
Kerdiles, Y.M. et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat. Immunol. 10, 176–184 (2009).
Kerdiles, Y.M. et al. Foxo transcription factors control regulatory T cell development and function. Immunity 33, 890–904 (2010).
Ouyang, W., Beckett, O., Flavell, R.A. & Li, M.O. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity 30, 358–371 (2009).
Ouyang, W. et al. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat. Immunol. 11, 618–627 (2010).
Carlson, C.M. et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 442, 299–302 (2006).
Takada, K. et al. Kruppel-like factor 2 is required for trafficking but not quiescence in postactivated T cells. J. Immunol. 186, 775–783 (2011).
Jones, R.G. & Thompson, C.B. Revving the engine: signal transduction fuels T cell activation. Immunity 27, 173–178 (2007).
Pearce, E.L. Metabolism in T cell activation and differentiation. Curr. Opin. Immunol. 22, 314–320 (2010).
Zoncu, R., Efeyan, A. & Sabatini, D.M. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 (2011).
Weichhart, T. & Saemann, M.D. The multiple facets of mTOR in immunity. Trends Immunol. 30, 218–226 (2009).
Powell, J.D. & Delgoffe, G.M. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity 33, 301–311 (2010).
Finlay, D. & Cantrell, D.A. Metabolism, migration and memory in cytotoxic T cells. Nat. Rev. Immunol. 11, 109–117 (2011).
Kwiatkowski, D.J. et al. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum. Mol. Genet. 11, 525–534 (2002).
Tait, S.W. & Green, D.R. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 11, 621–632 (2010).
Pendergrass, W., Wolf, N. & Poot, M. Efficacy of MitoTracker Green and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytometry A 61, 162–169 (2004).
Sinclair, L.V. et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat. Immunol. 9, 513–521 (2008).
Liu, G., Yang, K., Burns, S., Shrestha, S. & Chi, H. The S1P1-mTOR axis directs the reciprocal differentiation of TH1 and Treg cells. Nat. Immunol. 11, 1047–1056 (2010).
Hildeman, D.A. et al. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity 10, 735–744 (1999).
Menon, S. et al. COP9 signalosome subunit 8 is essential for peripheral T cell homeostasis and antigen receptor-induced entry into the cell cycle from quiescence. Nat. Immunol. 8, 1236–1245 (2007).
Boyman, O., Cho, J.H., Tan, J.T., Surh, C.D. & Sprent, J. A major histocompatibility complex class I-dependent subset of memory phenotype CD8+ cells. J. Exp. Med. 203, 1817–1825 (2006).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Lee, K. et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 32, 743–753 (2010).
Delgoffe, G.M. et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12, 295–303 (2011).
Jones, R.G. et al. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-XL levels in vivo. J. Exp. Med. 191, 1721–1734 (2000).
Inoki, K., Zhu, T. & Guan, K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590 (2003).
Manning, B.D., Tee, A.R., Logsdon, M.N., Blenis, J. & Cantley, L.C. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell 10, 151–162 (2002).
Weichhart, T. et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 29, 565–577 (2008).
Gan, B. et al. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc. Natl. Acad. Sci. USA 105, 19384–19389 (2008).
Chen, C. et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J. Exp. Med. 205, 2397–2408 (2008).
Choo, A.Y. et al. Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol. Cell 38, 487–499 (2010).
Ghosh, S. et al. Essential role of tuberous sclerosis genes TSC1 and TSC2 in NF-κB activation and cell survival. Cancer Cell 10, 215–226 (2006).
Rathmell, J.C., Farkash, E.A., Gao, W. & Thompson, C.B. IL-7 enhances the survival and maintains the size of naive T cells. J. Immunol. 167, 6869–6876 (2001).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009).
Pearce, E.L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
Rao, R.R., Li, Q., Odunsi, K. & Shrikant, P.A. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity 32, 67–78 (2010).
Delgoffe, G.M. et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30, 832–844 (2009).
Liu, G. et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat. Immunol. 10, 769–777 (2009).
Houtkooper, R.H., Williams, R.W. & Auwerx, J. Metabolic networks of longevity. Cell 142, 9–14 (2010).
Shiota, C., Woo, J.T., Lindner, J., Shelton, K.D. & Magnuson, M.A. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev. Cell 11, 583–589 (2006).
Huang, d.W., Sherman, B.T. & Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Acknowledgements
We thank P. Ohashi (University of Toronto) for Akt1-transgenic mice; T. Ludwig (Columbia University) for Rosa26-Cre-ERT2 mice; R. Cross, G. Lennon and S. Morgan for cell sorting; and the National Institutes of Health Tetramer Facility for the CD1d-PBS57 tetramer. Supported by the US National Institutes of Health (K01 AR053573 and R01 NS064599), the Arthritis Foundation, the Lupus Research Institute and the American Lebanese Syrian Associated Charities (H.C.).
Author information
Authors and Affiliations
Contributions
K.Y. designed and did cellular, molecular and biochemical experiments and contributed to the writing of the manuscript; G.N. did bioinformatic analyses; D.R.G. contributed genetic models and conceptual insights; W.H. contributed to cell purification; and H.C. designed experiments, wrote the manuscript and provided overall direction.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 and Tables 1–2 (PDF 1870 kb)
Rights and permissions
About this article
Cite this article
Yang, K., Neale, G., Green, D. et al. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol 12, 888–897 (2011). https://doi.org/10.1038/ni.2068
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2068
This article is cited by
-
Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease
Signal Transduction and Targeted Therapy (2023)
-
SEL1L preserves CD8+ T-cell survival and homeostasis by fine-tuning PERK signaling and the IL-15 receptor-mediated mTORC1 axis
Cellular & Molecular Immunology (2023)
-
Metabolic glycan labeling immobilizes dendritic cell membrane and enhances antitumor efficacy of dendritic cell vaccine
Nature Communications (2023)
-
Immunotherapy targeting plasma ASM is protective in a mouse model of Alzheimer’s disease
Nature Communications (2023)
-
PTEN directs developmental and metabolic signaling for innate-like T cell fate and tissue homeostasis
Nature Cell Biology (2022)