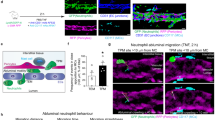
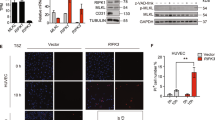
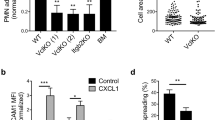
Abstract
The migration of neutrophils into inflamed tissues is a fundamental component of innate immunity. A decisive step in this process is the polarized migration of blood neutrophils through endothelial cells (ECs) lining the venular lumen (transendothelial migration (TEM)) in a luminal-to-abluminal direction. By real-time confocal imaging, we found that neutrophils had disrupted polarized TEM ('hesitant' and 'reverse') in vivo. We noted these events in inflammation after ischemia-reperfusion injury, characterized by lower expression of junctional adhesion molecule C (JAM-C) at EC junctions, and they were enhanced by blockade or genetic deletion of JAM-C in ECs. Our results identify JAM-C as a key regulator of polarized neutrophil TEM in vivo and suggest that reverse TEM of neutrophils can contribute to the dissemination of systemic inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ley, K., Laudanna, C., Cybulsky, M.I. & Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 (2007).
Nourshargh, S., Hordijk, P.L. & Sixt, M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell Biol. 11, 366–378 (2010).
Hurley, J.V. An electron microscopic study of leucocytic emigration and vascular permeability in rat skin. Aust. J. Exp. Biol. Med. Sci. 41, 171–186 (1963).
Marchesi, V.T. & Florey, V.T. Electron micrographic observations on the emigration of leucocytes. Q. J. Exp. Physiol. Cogn. Med. Sci. 45, 343–348 (1960).
Muller, W.A. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 24, 327–334 (2003).
Feng, D., Nagy, J.A., Pyne, K., Dvorak, H.F. & Dvorak, A.M. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J. Exp. Med. 187, 903–915 (1998).
Carman, C.V. et al. Transcellular diapedesis is initiated by invasive podosomes. Immunity 26, 784–797 (2007).
Cinamon, G., Shinder, V., Shamri, R. & Alon, R. Chemoattractant signals and β2 integrin occupancy at apical endothelial contacts combine with shear stress signals to promote transendothelial neutrophil migration. J. Immunol. 173, 7282–7291 (2004).
Mamdouh, Z., Mikhailov, A. & Muller, W.A. Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. J. Exp. Med. 206, 2795–2808 (2009).
Millan, J. et al. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat. Cell Biol. 8, 113–123 (2006).
Nieminen, M. et al. Vimentin function in lymphocyte adhesion and transcellular migration. Nat. Cell Biol. 8, 156–162 (2006).
Yang, L. et al. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-α-activated vascular endothelium under flow. Blood 106, 584–592 (2005).
Marmon, S., Cammer, M., Raine, C.S. & Lisanti, M.P. Transcellular migration of neutrophils is a quantitatively significant pathway across dermal microvascular endothelial cells. Exp. Dermatol. 18, 88–90 (2009).
Christofidou-Solomidou, M., Nakada, M.T., Williams, J., Muller, W.A. & DeLisser, H.M. Neutrophil platelet endothelial cell adhesion molecule-1 participates in neutrophil recruitment at inflammatory sites and is down-regulated after leukocyte extravasation. J. Immunol. 158, 4872–4878 (1997).
Faust, N., Varas, F., Kelly, L.M., Heck, S. & Graf, T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 96, 719–726 (2000).
Thompson, R.D. et al. Platelet-endothelial cell adhesion molecule-1 (PECAM-1)-deficient mice demonstrate a transient and cytokine-specific role for PECAM-1 in leukocyte migration through the perivascular basement membrane. Blood 97, 1854–1860 (2001).
Jung, S. et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Dejana, E. Endothelial cell-cell junctions: happy together. Nat. Rev. Mol. Cell Biol. 5, 261–270 (2004).
Muller, W.A. Mechanisms of transendothelial migration of leukocytes. Circ. Res. 105, 223–230 (2009).
Bradfield, P.F. et al. JAM-C regulates unidirectional monocyte transendothelial migration in inflammation. Blood 110, 2545–2555 (2007).
Scheiermann, C. et al. Junctional adhesion molecule-C mediates leukocyte infiltration in response to ischemia reperfusion injury. Arterioscler. Thromb. Vasc. Biol. 29, 1509–1515 (2009).
Lamagna, C. et al. Dual interaction of JAM-C with JAM-B and αMβ2 integrin: function in junctional complexes and leukocyte adhesion. Mol. Biol. Cell 16, 4992–5003 (2005).
Nourshargh, S., Krombach, F. & Dejana, E. The role of JAM-A and PECAM-1 in modulating leukocyte infiltration in inflamed and ischemic tissues. J. Leukoc. Biol. 80, 714–718 (2006).
Santoso, S. et al. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J. Exp. Med. 196, 679–691 (2002).
Chavakis, T. et al. The junctional adhesion molecule-C promotes neutrophil transendothelial migration in vitro and in vivo. J. Biol. Chem. 279, 55602–55608 (2004).
Tsukamoto, T., Chanthaphavong, R.S. & Pape, H.C. Current theories on the pathophysiology of multiple organ failure after trauma. Injury 41, 21–26 (2010).
Buckley, C.D. et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J. Leukoc. Biol. 79, 303–311 (2006).
Burns, A.R. et al. Neutrophil transendothelial migration is independent of tight junctions and occurs preferentially at tricellular corners. J. Immunol. 159, 2893–2903 (1997).
Sumagin, R. & Sarelius, I.H. Intercellular adhesion molecule-1 enrichment near tricellular endothelial junctions is preferentially associated with leukocyte transmigration and signals for reorganization of these junctions to accommodate leukocyte passage. J. Immunol. 184, 5242–5252 (2010).
Hoshi, O. & Ushiki, T. Scanning electron microscopic studies on the route of neutrophil extravasation in the mouse after exposure to the chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine (fMLP). Arch. Histol. Cytol. 62, 253–260 (1999).
Marmon, S. et al. Caveolin-1 expression determines the route of neutrophil extravasation through skin microvasculature. Am. J. Pathol. 174, 684–692 (2009).
Randolph, G.J. & Furie, M.B. Mononuclear phagocytes egress from an in vitro model of the vascular wall by migrating across endothelium in the basal to apical direction: role of intercellular adhesion molecule 1 and the CD11/CD18 integrins. J. Exp. Med. 183, 451–462 (1996).
Mathias, J.R. et al. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol. 80, 1281–1288 (2006).
Yoo, S.K. & Huttenlocher, A. Spatiotemporal photolabeling of neutrophil trafficking during inflammation in live zebrafish. J. Leukoc. Biol. 89, 661–667 (2011).
Bradfield, P.F., Nourshargh, S., Aurrand-Lions, M. & Imhof, B.A. JAM family and related proteins in leukocyte migration. Arterioscler. Thromb. Vasc. Biol. 27, 2104–2112 (2007).
Orlova, V.V., Economopoulou, M., Lupu, F., Santoso, S. & Chavakis, T. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell-cell contacts. J. Exp. Med. 203, 2703–2714 (2006).
Mulero, V., Sepulcre, M.P., Rainger, G.E. & Buckley, C. Neutrophils live on a two-way street. J. Leukoc. Biol. 89, 645–647 (2011).
Aurrand-Lions, M. et al. Junctional adhesion molecule-C regulates the early influx of leukocytes into tissues during inflammation. J. Immunol. 174, 6406–6415 (2005).
Acknowledgements
We thank E. Dejana (FIRC Institute of Molecular Oncology) for mAb BV11 to JAM-A; S.J. Weiss for critical reading of the manuscript; R. Yadav for advice on surgical procedures; and H.M. McGetterick for contributions to analysis of rTEM neutrophils generated in vitro. Supported by the Wellcome Trust (081172/Z/06/Z to S.N.), the UK National Institute for Health Research (for the translational research portfolio of Barts and the London Cardiovascular Biomedical Research Unit, to which the work of S.N. contributes), the Swiss National Science Foundation (310000-122423, 310000-109402 and CR32I3_129987 to P.M. and 310030-120184 for B.A.I.), the Juvenile Diabetes Research Foundation (40-2011-11 to P.M.), the European Union (BETAIMAGE 222980; IMIDIA and C2008-T7 to P.M.; and a Marie Curie Fellowship (FP7-PEOPLE-2009-IEF-252091) to M.B.), the Intramural Research Program of the US National Institutes of Health (T.C.), the National Cancer Institute (T.C.) and Deutsche Forschungsgemeinschaft (T.C.).
Author information
Authors and Affiliations
Contributions
A.W. designed and did most experiments, analyzed data and contributed to the writing of the manuscript; M.-B.V. designed and did the immunofluorescence staining experiments and contributed to method development and data analysis and interpretation; M.B. designed and did flow cytometry assays and contributed to data analysis and interpretation; B.C., D.C. and F.-M.D. designed and did some assays; G.B. T.C., S.M.A., G.E.R. and P.M. provided reagents and/or contributed to the design of experiments; B.A.I. provided reagents and made intellectual contributions to the study; and S.N. provided overall project supervision and contributed to the design of the experiments and the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Methods and Supplementary Results (PDF 752 kb)
Supplementary Video 1
Development of an inflammatory response in an IL-1β-stimulated tissue. (WMV 1553 kb)
Supplementary Video 2
Migration of leukocytes in a paracellular mode. (WMV 1172 kb)
Supplementary Video 3
Paracellular transmigration and pore formation (WMV 1058 kb)
Supplementary Video 4
Transcellular TEM and pore formation (WMV 1067 kb)
Supplementary Video 5
Hesitant TEM as induced by I-R injury (example 1). (WMV 1373 kb)
Supplementary Video 6
Hesitant TEM as induced by I-R injury (example 2). (WMV 1259 kb)
Supplementary Video 7
Hesitant TEM as induced by I-R injury (example 3). (WMV 917 kb)
Supplementary Video 8
Reverse TEM as induced by I-R injury. (WMV 1847 kb)
Rights and permissions
About this article
Cite this article
Woodfin, A., Voisin, MB., Beyrau, M. et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol 12, 761–769 (2011). https://doi.org/10.1038/ni.2062
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2062
This article is cited by
-
Pathological hemodynamic changes and leukocyte transmigration disrupt the blood–spinal cord barrier after spinal cord injury
Journal of Neuroinflammation (2023)
-
Strategies of neutrophil diversification
Nature Immunology (2023)
-
Effects of neutrophil fate on inflammation
Inflammation Research (2023)
-
Nectins and Nectin-like molecules drive vascular development and barrier function
Angiogenesis (2023)
-
Recent advances in the role of neutrophils and neutrophil extracellular traps in acute pancreatitis
Clinical and Experimental Medicine (2023)