Abstract
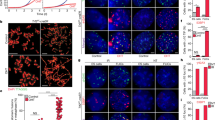
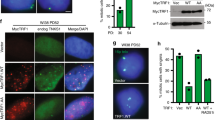
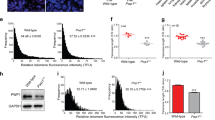
The molecular mechanisms of cellular mortality have recently begun to be unraveled. In particular, it has been discovered that cells that lack telomerase are subject to telomere attrition with each round of replication, eventually leading to loss of telomere capping function at chromosome ends1,2,3,4,5. Critically short telomeres and telomeres lacking telomere-binding proteins lose their functionality and are metabolized as DNA breaks, thus generating chromosomal fusions1,2,3,4,5,6,7,8. Telomerase activity is sufficient to rescue short telomeres and confers an unlimited proliferative capacity9,10,11. In addition, the tumor-suppressor pathway Cdkn2a/Rb1 has also been implicated as a barrier to immortalization12,13,14. Here, we report a connection between the members of the retinoblastoma family of proteins, Rb1 (retinoblastoma 1), Rbl1 (retinoblastoma-like 1) and Rbl2 (retinoblastoma-like 2), and the mechanisms that regulate telomere length. In particular, mouse embryonic fibroblasts doubly deficient in Rbl1 and Rbl2 or triply deficient in Rbl1, Rbl2 and Rb1 have markedly elongated telomeres compared with those of wildtype or Rb1-deficient cells. This deregulation of telomere length is not associated with increased telomerase activity. Notably, the abnormally elongated telomeres in doubly or triply deficient cells retain their end-capping function, as shown by the normal frequency of chromosomal fusions. These findings demonstrate a connection between the Rb1 family and the control of telomere length in mammalian cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Blackburn, E.H. Switching and signaling at the telomere. Cell 106, 661–673 (2001).
Chan, S.W.-L. & Blackburn, E.H. New ways not to make ends meet: telomerase, DNA damage proteins and heterochromatin. Oncogene 21, 553–563 (2002).
de Lange, T. Protection of mammalian telomeres. Oncogene 21, 532–540 (2002).
Goytisolo, F.A. & Blasco, M.A. Many ways to telomere dysfunction: in vivo studies using mouse models. Oncogene 21, 584–591 (2002).
Harley, C.B., Futcher, A.B. & Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 31, 458–460 (1990).
Collins, K. & Mitchell, J.R. Telomerase in the human organism. Oncogene 21, 564–579 (2002).
Blasco, M.A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 (1997).
Lee, H.-W. et al. Essential role of mouse telomerase in highly proliferative organs. Nature 392, 569–574 (1998).
Samper, E., Flores, J.M. & Blasco, M.A. Restoration of telomerase activity rescues chromosomal instability and premature aging in Terc−/− mice with short telomeres. EMBO Reports 2, 800–807 (2001).
Hemann, M.T., Strong, M.A., Hao, L.Y. & Greider, C.W. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 107, 67–77 (2001).
Bodnar, A.G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998).
Kiyono, T. et al. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396, 84–88 (1998).
Farwell, D.G. et al. Genetic and epigenetic changes in human epithelial cells immortalized by telomerase. Am. J. Pathol. 156, 1537–1547 (2000).
Stoppler, H., Hartmann, D.P., Sherman, L. & Schlegel, R. The human papillomavirus type 16 E6 and E7 oncoproteins dissociate cellular telomerase activity from the maintenance of telomere length. J. Biol. Chem. 272, 13332–13337 (1997).
Sage, J. et al. Targeted disruption of the three Rb-related genes leads to loss of G1 control and immortalization. Genes Dev. 14, 3037–3050 (2000).
van Steensel, B., Smogorzewska, A. & de Lange, T. TRF2 protects human telomeres from end-to-end fusions. Cell 92, 401–413 (1998).
Samper, E., Goytisolo, F., Slijepcevic, P., van Buul, P. & Blasco, M.A. Mammalian Ku86 prevents telomeric fusions independently of the length of TTAGGG repeats and the G-strand overhang. EMBO Reports 1, 244–252 (2000).
Goytisolo, F., Samper, E., Edmonson, S., Taccioli, G.E. & Blasco, M.A. Absence of DNA-PKcs in mice results in anaphase bridges and in increased telomeric fusions with normal telomere length and G-strand overhang. Mol. Cell. Biol. 21, 3642–3651 (2001).
Hande, P.M., Samper, E., Lansdorp, P. & Blasco, M.A. Telomere length dynamics in cultured cells from normal and telomerase-null mice. J. Cell Biol. 144, 589–601 (1999).
Chong, L. et al. A human telomeric protein. Science 270, 1663–1667 (1995).
Smogorzewska, A. et al. Control of human telomere length by TRF1 and TRF2. Mol. Cell. Biol. 20, 1659–1668 (2000).
van Steensel, B. & de Lange, T. Control of telomere length by the human telomeric protein TRF1. Nature 385, 740–743 (1997).
Espejel, S. et al. Mammalian Ku86 mediates chromosomal fusions and apoptosis caused by critically short telomeres. EMBO J. 21, 2207–2219 (2002).
Henson, J.D., Neumann, A.A., Yeager, T.R. & Reddel, R.R. Alternative lengthening of telomeres in mammalian cells. Oncogene 21, 598–610 (2002).
Perrem, K., Colgin, L.M., Neumann, A.A., Yeager, T.R. & Reddel, R.R. Co-existence of alternative lengthening of telomeres and telomerase in hTERT-transfected GM847 cells. Mol. Cell. Biol. 21, 3862–3875 (2001).
Murga, M. et al. Mutation of the E2F2 in mice causes enhanced T lymphocyte proliferation, leading to the development of autoimmunity. Immunity 15, 959–970 (2001).
McIlrath, J.S. et al. Telomere length abnormalities in mammalian radiosensitive cells. Cancer Res. 61, 912–915 (2001).
Herrera, E. et al. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 18, 2950–2960 (1999).
Zijlmans, J.M. et al. Telomeres in the mouse have large inter-chromosomal variations in the number of T2AG3 repeats. Proc. Natl Acad. Sci. USA 94, 7423–7428 (1997).
Acknowledgements
We thank T. Jacks and J. Sage for the donation of numerous vials of TKO and DKO MEFs, B. Deberman and S. Weintraub for the gift of Rb1−/− and Rb1+/+ MEFs and M. Serrano for critical reading of the manuscript and helpful discussions. M.G.-C. is a predoctoral fellow from the Spanish Ministry of Science and Technology. S.G. is supported by the US National Institutes of Health. Research at the laboratory of M.A.B. is funded by the Spanish Ministry of Science and Technology, the Regional Government of Madrid, the European Union and the Department of Immunology and Oncology (Spanish Research Council/Pharmacia Corporation).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
García-Cao, M., Gonzalo, S., Dean, D. et al. A role for the Rb family of proteins in controlling telomere length. Nat Genet 32, 415–419 (2002). https://doi.org/10.1038/ng1011
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1011
This article is cited by
-
The telomere complex and the origin of the cancer stem cell
Biomarker Research (2021)
-
RBL2 bi-allelic truncating variants cause severe motor and cognitive impairment without evidence for abnormalities in DNA methylation or telomeric function
Journal of Human Genetics (2021)
-
Pan-cancer analysis of whole genomes
Nature (2020)
-
Genomic characterization of chronic lymphocytic leukemia (CLL) in radiation-exposed Chornobyl cleanup workers
Environmental Health (2018)
-
TERRA recruitment of polycomb to telomeres is essential for histone trymethylation marks at telomeric heterochromatin
Nature Communications (2018)