Abstract
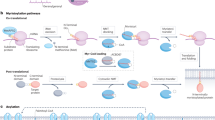
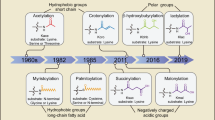
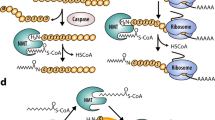
In eukaryotic cells, a specific set of proteins are modified by C-terminal attachment of 15-carbon farnesyl groups or 20-carbon geranylgeranyl groups that function both as anchors for fixing proteins to membranes and as molecular handles for facilitating binding of these lipidated proteins to other proteins. Additional modification of these prenylated proteins includes C-terminal proteolysis and methylation, and attachment of a 16-carbon palmitoyl group; these modifications augment membrane anchoring and alter the dynamics of movement of proteins between different cellular membrane compartments. The enzymes in the protein prenylation pathway have been isolated and characterized. Blocking protein prenylation is proving to be therapeutically useful for the treatment of certain cancers, infection by protozoan parasites and the rare genetic disease Hutchinson-Gilford progeria syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sakagami, Y. et al. Isolation of a novel sex hormone tremerogen A-10, controlling conjugation tube formation in Tremella mesenterica fries. Agric. Biol. Chem. 42, 1093–1094 (1978).
Tsuchiya, E., Fukui, S., Kamiya, Y., Sakagami, Y. & Fujino, M. Requirements of chemical structure for hormonal activity of lipo-peptidyl factors inducing sexual differentiation in vegetative cells of heterobasidiomycetous yeasts. Biochem. Biophys. Res. Commun. 85, 459–463 (1978).
Anderegg, R.J., Betz, R., Carr, S.A., Crabb, J.W. & Duntze, W. Structure of Saccharomyces cerevisiae mating hormone a-factor. Identification of S-farnesyl cysteine as a structural component. J. Biol. Chem. 263, 18236–18240 (1988).
Glomset, J.A., Gelb, M.H. & Farnsworth, C.C. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem. Sci. 15, 139–142 (1990).
Farnsworth, C.C., Wolda, S.L., Gelb, M.H. & Glomset, J.A. Human lamin B contains a farnesylated cysteine residue. J. Biol. Chem. 264, 20422–20429 (1989).
Farnsworth, C.C., Gelb, M.H. & Glomset, J.A. Identification of geranylgeranyl-modified proteins in HeLa cells. Science 247, 320–322 (1990).
Farnsworth, C.C., Seabra, M.C., Ericsson, L.H., Gelb, M.H. & Glomset, J.A. Rab geranylgeranyl transferase catalyzes the geranylgeranylation of adjacent cysteines in the small GTPases, Rab1A, Rab3A, and Rab5A. Proc. Natl. Acad. Sci. USA 91, 11963–11967 (1994).
Gelb, M.H., Reiss, Y., Ghomashchi, F. & Farnsworth, C.C. Exploring the specificity of prenyl protein-specific methyltranferase with synthetic prenylated rab peptides. Bioorg. Med. Chem. Lett. 8, 881–886 (1995).
Yokoyama, K., Goodwin, G.W., Ghomashchi, F., Glomset, J. & Gelb, M.H. Protein prenyltransferases. Biochem. Soc. Trans. 20, 489–494 (1992).
Casey, P.J. & Seabra, M.C. Protein prenyltransferases. J. Biol. Chem. 271, 5289–5292 (1996).
Taylor, J.S., Reid, T.S., Terry, K.L., Casey, P.J. & Beese, L.S. Structure of mammalian protein geranylgeranyltransferase type-I. EMBO J. 22, 5963–5974 (2003).
Long, S.B., Casey, P.J. & Beese, L.S. Reaction path of protein farnesyltransferase at atomic resolution. Nature 419, 645–650 (2002).
Zhang, H., Seabra, M.C. & Deisenhofer, J. Crystal structure of Rab geranylgeranyltransferase at 2 angstrom resolution. Structure 8, 241–251 (2000).
Hicks, K.A., Hartman, H.L. & Fierke, C.A. Upstream polybasic region in peptides enhances dual specificity for prenylation by both farnesyltransferase and geranylgeranyltransferase type I. Biochemistry 44, 15325–15333 (2005).
Yokoyama, K., Goodwin, G.W., Ghomashchi, F., Glomset, J.A. & Gelb, M.H. A protein geranylgeranyltransferase from bovine brain: implications for protein prenylation specificity. Proc. Natl. Acad. Sci. USA 88, 5302–5306 (1991).
Reid, T.S., Terry, K.L., Casey, P.J. & Beese, L.S. Crystallographic analysis of CaaX prenyltransferases complexed with substrates defines rules of protein substrate selectivity. J. Mol. Biol. 343, 417–433 (2004).
Long, S.B., Casey, P.J. & Beese, L.S. The basis for K-Ras4B binding specificity to protein farnesyltransferase revealed by 2 A resolution ternary complex structures. Structure 8, 209–222 (2000).
Leung, K.F., Baron, R. & Seabra, M.C. Thematic review series: lipid posttranslational modifications. Geranylgeranylation of Rab GTPases. J. Lipid Res. 47, 467–475 (2006).
Winter-Vann, A.M. & Casey, P.J. Post-prenylation-processing enzymes as new targets in oncogenesis. Nat. Rev. Cancer 5, 405–412 (2005).
Young, S.G., Clarke, S., Bergo, M.O., Philips, M. & Fong, L.G. in The Enzymes Vol. 24 (eds Clarke, S.G. & Tamanoi, F.) 273–301 (Academic/Elsevier, San Diego, 2006).
Tam, A. et al. Dual roles for Ste24p in yeast a-factor maturation: NH2-terminal proteolysis and COOH-terminal CAAX processing. J. Cell Biol. 142, 635–649 (1998).
Boyartchuk, V.L., Ashby, M.N. & Rine, J. Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science 275, 1796–1800 (1997).
Trueblood, C.E. et al. The CaaX proteases, Afc1p and Rce1p, have overlapping but distinct substrate specificities. Mol. Cell. Biol. 20, 4381–4392 (2000).
Schmidt, W.K., Tam, A., Fujimura-Kamada, K. & Michaelis, S. Endoplasmic reticulum membrane localization of Rce1p and Ste24p, yeast proteases involved in carboxyl-terminal CAAX protein processing and amino-terminal a-factor cleavage. Proc. Natl. Acad. Sci. USA 95, 11175–11180 (1998).
Tam, A., Schmidt, W.K. & Michaelis, S. The multispanning membrane protein Ste24p catalyzes CAAX proteolysis and NH2-terminal processing of the yeast a-factor precursor. J. Biol. Chem. 276, 46798–46806 (2001).
Ma, Y.T., Chaudhuri, A. & Rando, R.R. Substrate specificity of the isoprenylated protein endoprotease. Biochemistry 31, 11772–11777 (1992).
Ma, Y.T., Gilbert, B.A. & Rando, R.R. Inhibitors of the isoprenylated protein endoprotease. Biochemistry 32, 2386–2393 (1993).
Jang, G.F. & Gelb, M.H. Substrate specificity of mammalian prenyl protein-specific endoprotease activity. Biochemistry 37, 4473–4481 (1998).
Dolence, J.M., Steward, L.E., Dolence, E.K., Wong, D.H. & Poulter, C.D. Studies with recombinant Saccharomyces cerevisiae CaaX prenyl protease Rce1p. Biochemistry 39, 4096–4104 (2000).
Chen, Y., Ma, Y.T. & Rando, R.R. Solubilization, partial purification, and affinity labeling of the membrane-bound isoprenylated protein endoprotease. Biochemistry 35, 3227–3237 (1996).
Otto, J.C. et al. Cloning and characterization of a mammalian prenyl protein-specific endoprtease. J. Biol. Chem. 274, 8379–8382 (1999).
Pei, J. & Grishin, N.V. Type II CAAX prenyl endopeptidases belong to a novel superfamily of putative membrane-bound metalloproteases. Trends Biochem. Sci. 26, 275–277 (2001).
Plummer, L.J. et al. Mutational analysis of the ras converting enzyme reveals a requirement for glutamate and histidine residues. J. Biol. Chem. 281, 4596–4605 (2006).
Bergo, M.O. et al. On the physiological importance of endoproteolysis of CAAX proteins: heart-specific RCE1 knockout mice develop a lethal cardiomyopathy. J. Biol. Chem. 279, 4729–4736 (2004).
Kim, E. et al. Disruption of the mouse Rce1 gene results in defective Ras processing and mislocalization of Ras within cells. J. Biol. Chem. 274, 8383–8390 (1999).
Bergo, M.O. et al. Absence of the CAAX endoprotease Rce1: effects on cell growth and transformation. Mol. Cell. Biol. 22, 171–181 (2002).
Schlitzer, M., Winter-Vann, A. & Casey, P.J. Non-peptidic, non-prenylic inhibitors of the prenyl protein-specific protease Rce1. Bioorg. Med. Chem. Lett. 11, 425–427 (2001).
Romano, J.D., Schmidt, W.K. & Michaelis, S. The Saccharomyces cerevisiae prenylcysteine carboxyl methyltransferase Ste14p is in the endoplasmic reticulum membrane. Mol. Biol. Cell 9, 2231–2247 (1998).
Romano, J.D. & Michaelis, S. Topological and mutational analysis of Saccharomyces cerevisiae Ste14p, founding member of the isoprenylcysteine carboxyl methyltransferase family. Mol. Biol. Cell 12, 1957–1971 (2001).
Anderson, J.L. & Hrycyna, C.A. in The Enzymes Vol. 24 (eds Clarke, S.G. & Tamanoi, F.) 245–272 (Academic/Elsevier, San Diego, 2006).
Anderson, J.L., Frase, H., Michaelis, S. & Hrycyna, C.A. Purification, functional reconstitution, and characterization of the Saccharomyces cerevisiae isoprenylcysteine carboxylmethyltransferase Ste14p. J. Biol. Chem. 280, 7336–7345 (2005).
Bergo, M.O. et al. Isoprenylcysteine carboxyl methyltransferase deficiency in mice. J. Biol. Chem. 276, 5841–5845 (2001).
Bergo, M.O. et al. Targeted inactivation of the isoprenylcysteine carboxyl methyltransferase gene causes mislocalization of K-Ras in mammalian cells. J. Biol. Chem. 275, 17605–17610 (2000).
Bergo, M.O. et al. Inactivation of Icmt inhibits transformation by oncogenic K-Ras and B-Raf. J. Clin. Invest. 113, 539–550 (2004).
Sapperstein, S., Berkower, C. & Michaelis, S. Nucleotide sequence of the yeast STE14 gene, which encodes farnesylcysteine carboxyl methyltransferase, and demonstration of its essential role in a-factor export. Mol. Cell. Biol. 14, 1438–1449 (1994).
Wright, L.P. & Philips, M.R. CAAX modification and membrane targeting of Ras. J. Lipid Res. 47, 883–891 (2006).
Philips, M.R. Methotrexate and Ras methylation: a new trick for an old drug? Sci. STKE 2004, pe13 (2004).
Winter-Vann, A.M. et al. Targeting Ras signaling through inhibition of carboxyl methylation: an unexpected property of methotrexate. Proc. Natl. Acad. Sci. USA 100, 6529–6534 (2003).
Michaelson, D. et al. Postprenylation CAAX processing is required for proper localization of Ras but not Rho GTPases. Mol. Biol. Cell 16, 1606–1616 (2005).
Winter-Vann, A.M. et al. A small-molecule inhibitor of isoprenylcysteine carboxyl methyltransferase with antitumor activity in cancer cells. Proc. Natl. Acad. Sci. USA 102, 4336–4341 (2005).
Anderson, J.L., Henriksen, B.S., Gibbs, R.A. & Hrycyna, C.A. The isoprenoid substrate specificity of isoprenylcysteine carboxylmethyltransferase: development of novel inhibitors. J. Biol. Chem. 280, 29454–29461 (2005).
Smotrys, J.E. & Linder, M.E. Palmitoylation of intracellular signaling proteins: regulation and function. Annu. Rev. Biochem. 73, 559–587 (2004).
Dietrich, L.E.P. & Ungermann, C. On the mechanism of protein palmitoylation. EMBO Rep. 5, 1053–1057 (2004).
Linder, M.E. & Deschenes, R.J. New insights into the mechanisms of protein palmitoylation. Biochemistry 42, 4311–4320 (2003).
Hancock, J.F. Ras proteins: different signals from different locations. Nat. Rev. Mol. Cell Biol. 4, 373–384 (2003).
Webb, Y., Hermida-Matsumoto, L. & Resh, M.D. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J. Biol. Chem. 275, 261–270 (2000).
Lawrence, D.S., Zilfou, J.T. & Smith, C.D. Structure-activity studies of cerulenin analogues as protein palmitoylation inhibitors. J. Med. Chem. 42, 4932–4941 (1999).
Reents, R., Wagner, M., Kuhlmann, J. & Waldmann, H. Synthesis and application of fluorescence-labeled Ras-proteins for live-cell imaging. Angew. Chem. Int. Edn Engl. 43, 2711–2714 (2004).
Bartels, D.J., Mitchell, D.A., Dong, X.W. & Deschenes, R.J. Erf2, a novel gene product that affects the localization and palmitoylation of Ras2 in Saccharomyces cerevisiae. Mol. Cell. Biol. 19, 6775–6787 (1999).
Lobo, S., Greentree, W.K., Linder, M.E. & Deschenes, R.J. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 277, 41268–41273 (2002).
Swarthout, J.T. et al. DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. J. Biol. Chem. 280, 31141–31148 (2005).
Deck, P. et al. Development and biological evaluation of acyl protein thioesterase 1 (APT1) inhibitors. Angew. Chem. Int. Edn Engl. 44, 4975–4980 (2005).
Duncan, J.A. & Gilman, A.G. A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21(RAS). J. Biol. Chem. 273, 15830–15837 (1998).
Bader, B. et al. Bioorganic synthesis of lipid-modified proteins for the study of signal transduction. Nature 403, 223–226 (2000).
Dudler, T. & Gelb, M.H. Palmitoylation of Ha-Ras facilitates membrane binding, activation of downstream effectors, and meiotic maturation in Xenopus oocytes. J. Biol. Chem. 271, 11541–11547 (1996).
Silvius, J.R. Mechanisms of Ras protein targeting in mammalian cells. J. Membr. Biol. 190, 83–92 (2002).
Rocks, O. et al. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science 307, 1746–1752 (2005).
Goodwin, J.S. et al. Depalmitoylated Ras traffics to and from the Golgi complex via a nonvesicular pathway. J. Cell Biol. 170, 261–272 (2005).
Roy, S. et al. Individual palmitoyl residues serve distinct roles in H-ras trafficking, microlocalization, and signaling. Mol. Cell. Biol. 25, 6722–6733 (2005).
Wittinghofer, A. & Waldmann, H. Ras - a molecular switch involved in tumor formation. Angew. Chem. Int. Edn Engl. 39, 4193–4214 (2000).
Edidin, M. The state of lipid rafts: from model membranes to cells. Annu. Rev. Biophys. Biomol. Struct. 32, 257–283 (2003).
Silvius, J.R. Lipidated peptides as tools for understanding the membrane interactions of lipid-modified proteins. Peptide-Lipid Interactions 52, 371–395 (2002).
Hancock, J.F. & Parton, R.G. Ras plasma membrane signalling platforms. Biochem. J. 389, 1–11 (2005).
Wang, T.Y., Leventis, R. & Silvius, J.R. Partitioning of lipidated peptide sequences into liquid-ordered lipid domains in model and biological membranes. Biochemistry 40, 13031–13040 (2001).
Zacharias, D.A., Violin, J.D., Newton, A.C. & Tsien, R.Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296, 913–916 (2002).
Nicolini, C. et al. Visualizing association of N-Ras in lipid microdomains: influence of domain structure and interfacial adsorption. J. Am. Chem. Soc. 128, 192–201 (2006).
Reuther, G. et al. Structural model of the membrane-bound C terminus of lipid-modified human N-Ras protein. Angew. Chem. Int. Edn Engl. 45, 5387–5390 (2006).
Rotblat, B. et al. Three separable domains regulate GTP-dependent association of H-ras with the plasma membrane. Mol. Cell. Biol. 24, 6799–6810 (2004).
Plowman, S.J., Muncke, C., Parton, R.G. & Hancock, J.F. H-ras, K-ras, and inner plasma membrane raft proteins operate in nanoclusters with differential dependence on the actin cytoskeleton. Proc. Natl. Acad. Sci. USA 102, 15500–15505 (2005).
Plowman, S.J. & Hancock, J.F. Ras signaling from plasma membrane and endomembrane microdomains. Biochimica et Biophysica Acta-Molecular Cell Research 1746, 274–283 (2005).
Hancock, J.F., Cadwallader, K., Paterson, H. & Marshall, C.J.A. CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 10, 4033–4039 (1991).
Okeley, N.M. & Gelb, M.H. A designed probe for acidic phospholipids reveals the unique enriched anionic character of the cytosolic face of the mammalian plasma membrane. J. Biol. Chem. 279, 21833–21840 (2004).
Ishizaki, H. et al. Role of rab GDP dissociation inhibitor alpha in regulating plasticity of hippocampal neurotransmission. Proc. Natl. Acad. Sci. USA 97, 11587–11592 (2000).
DerMardirossian, C. & Bokoch, G.M. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 15, 356–363 (2005).
Dovas, A. & Couchman, J.R. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem. J. 390, 1–9 (2005).
Hoffman, G.R., Nassar, N. & Cerione, R.A. Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell 100, 345–356 (2000).
Pylypenko, O. et al. Structure of doubly prenylated Ypt1:GDI complex and the mechanism of GDI mediated Rab:membrane interaction. EMBO J. 25, 13–23 (2006).
Pfeffer, S. & Aivazian, D. Targeting RAB GTPases to distinct membrane compartments. Nat. Rev. Mol. Cell Biol. 5, 886–896 (2004).
Nilsson, B.L., Soellner, M.B. & Raines, R.T. Chemical synthesis of proteins. Annu. Rev. Biophys. Biomol. Struct. 34, 91–118 (2005).
Tamanoi, F. & Gau, C.L., Jiang, C., Edamatsu, H. & Kato-Stankiewicz, K. Protein farnesylation in mammalian cells: effects of farnesyltransferase inhibitors on cancer cells. Cell. Mol. Life Sci. 58, 1636–1649 (2001).
Basso, A., Kirshmeier, P. & Bishop, W.R. Farnesyltransferase inhibitors. J. Lipid Res. 47, 15–31 (2006).
Reid, T.S. & Beese, L.S. Crystal structures of the anticancer clinical candidates R115777 (Tipifarnib) and BMS-214662 complexed with protein farnesyltransferase suggest a mechanism of FTI selectivity. Biochemistry 43, 6877–6884 (2004).
Lobell, R.B. et al. Preclinial and clinical pharmacodynamic assessment of L-778,123, a dual inhibitor of farnesyl:protein transferase and geranylgeranyl:protein transferase type-I. Mol. Cancer Ther. 1, 747–758 (2002).
Reid, T.S., Long, S.B. & Beese, L.S. Crystallographic analysis reveals that anticancer clinical candidate L-778,123 inhibits protein farnesyltransferase and geranylgeranyltransferase-I by different binding modes. Biochemistry 43, 9000–9008 (2004).
Lackner, M.R. et al. Chemical genetics identifies Rab geranylgeranyl transferase as an apoptotic target of farnesyl transferase inhibitors. Cancer Cell 7, 325–336 (2005).
Karp, J.E. et al. Clinical and biologic activity of the farnesyltransferase inhibitor R115777 in adults with refractory and relapsed acute leukemia: a phase I clinical-laboratory correlative trial. Blood 97, 3361–3369 (2001).
Alsina, M. et al. Farnesyltransferase inhibitor tipifarnib is well tolerated, induces stabilization of disease and inhibits farnesylation and oncogenic/tumor survival pathways in patients with advanced multiple myeloma. Blood 103, 3271–3277 (2004).
Khuri, F.R. et al. Phase I study of the farnesyltransferase inhibitor lonafarnib with paclitaxel in solid tumors. Clin. Cancer Res. 10, 2968–2976 (2004).
James, G.L., Goldstein, J.L. & Brown, M.S. Polylysine and CVIM sequences of K-RasB dictate specificity of prenylation and confer resistance to benzodiazepine peptidomimetic in vitro. J. Biol. Chem. 270, 6221–6226 (1995).
Whyte, D.B. et al. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J. Biol. Chem. 272, 14459–14464 (1997).
Yoo, J. & Robinson, R.A. H-ras gene mutations in salivary gland mucoepidermoid carcinomas. Cancer 88, 518–523 (2000).
Burchill, S.A., Neal, D.E. & Lunec, J. Frequency of H-ras mutations in human bladder cancer detected by direct sequencing. Br. J. Urol. 73, 516–521 (1994).
Aoki, Y. et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat. Genet. 37, 1038–1040 (2005).
Estep, A.L., Tidyman, W.E., Teitell, M.A., Cotter, P.D. & Rauen, K.A. HRAS mutations in Costello syndrome: detection of constitutional activating mutations in codon 12 and 13 and loss of wild-type allele in malignancy. Am. J. Med. Genet. A 140, 8–16 (2006).
Gripp, K.W. et al. HRAS mutation analysis in Costello syndrome: genotype and phenotype correlation. Am. J. Med. Genet. A 140, 1–7 (2006).
Gomez, M., Sampson, J. & Whittemore, V. The Tuberous Sclerosis Complex (Oxford University Press, Oxford, 1999).
Gau, C.-L. et al. Farnesyltransferase inhibitors reverse altered growth and distribution of actin filaments in Tsc-deficient cells via inhibition of both rapamycin-sensitive and –insensitive pathways. Mol. Cancer Ther. 4, 918–926 (2005).
Basso, A.D. et al. The farnesyltransferase inhibitor (FTI) SCH66336 (lonafarnib) inhibits Rheb farnesylation and mTOR signaling. Role in FTI enhancement of taxane and tamoxifen anti-tumor activity. J. Biol. Chem. 280, 31101–31108 (2005).
Urano, J. et al. Identification of novel single amino acid changes that result in hyperactivation of the unique GTPase, Rheb, in fission yeast. Mol. Microbiol. 58, 1074–1086 (2005).
Long, X., Lin, Y., Ortiz-Vega, S., Yonezawa, K. & Avruch, J. Rheb binds and regulates the mTOR kinase. Curr. Biol. 15, 702–713 (2005).
Carsillo, T., Astrindis, A. & Henske, E.P. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc. Natl. Acad. Sci. USA 97, 6085–6090 (2000).
Bardelli, A. et al. PRL-3 expression in metastatic cancers. Clin. Cancer Res. 9, 5607–5615 (2003).
Fiordalisi, J.J., Keller, P.J. & Cox, A.D. PRL tyrosine phosphatases regulate rho family GTPases to promote invasion and motility. Cancer Res. 66, 3153–3161 (2006).
Lim, K.-H. et al. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell 7, 533–545 (2005).
Hakem, A. et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 19, 1974–1979 (2005).
Clark, E.A., Golub, T., Lander, E.S. & Hynes, R.O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406, 532–535 (2000).
Carrico, D., Blaskovich, M.A., Bucher, C., Sebti, S. & Hamilton, A.D. Design, synthesis, and evaluation of potent and selective benzoyleneurea-based inhibitors of protein geranylgeranyltransferase-I. Bioorg. Med. Chem. 13, 677–688 (2005).
Peterson, Y.K., Kelly, P., Weinbaum, C.A. & Casey, P.J. A novel protein geranylgeranyltransferase-I inhibitor with high potency, selectivity and cellular activity. J. Biol. Chem. 281, 12445–12450 (2006).
Eriksson, M. et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423, 293–298 (2003).
Young, S.G., Fong, L.G. & Michaelis, S. Prelamin A, Zmpste24, misshapen cell nuclei, and progeria–new evidence suggesting that protein farnesylation could be important for disease pathogenesis. J. Lipid Res. 46, 2531–2558 (2005).
Bergo, M.O. et al. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc. Natl. Acad. Sci. USA 99, 13049–13054 (2002).
Pendas, A.M. et al. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat. Genet. 31, 94–99 (2002).
Fujimura-Kamada, K., Nouvet, F.J. & Michaelis, S. A novel membrane-associated metalloprotease, Ste24p, is required for the first step of NH2-terminal processing of the yeast a-factor precursor. J. Cell Biol. 136, 271–285 (1997).
Tam, A. et al. Dual roles for Ste24p in yeast a-factor maturation: NH2-terminal proteolysis and COOH-terminal CAAX processing. J. Cell Biol. 142, 635–649 (1998).
Capell, B.C. et al. Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 102, 12879–12884 (2005).
Glynn, M.W. & Glover, T.W. Incomplete processing of mutant lamin A in Hutchinson-Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase inhibition. Hum. Mol. Genet. 14, 2959–2969 (2005).
Goldman, R.D. et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 101, 8963–8968 (2004).
Mallampalli, M.P., Huyer, G., Bendale, P., Gelb, M.H. & Michaelis, S. Inhibiting farnesylation reverses the nuclear morphology defect in a HeLa cell model for Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 102, 14416–14421 (2005).
Toth, J.I. et al. Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc. Natl. Acad. Sci. USA 102, 12873–12878 (2005).
Yang, S.H. et al. Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson-Gilford progeria syndrome mutation. Proc. Natl. Acad. Sci. USA 102, 10291–10296 (2005).
Yang, S.H. et al. Farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation. J. Clin. Invest. 116, 2115–2121 (2006).
Fong, L.G. et al. A protein farnesyltransferase inhibitor ameliorates disease in a mouse model of progeria. Science 311, 1621–1623 (2006).
Buckner, F.S. et al. Cloning, heterologous expression, and distinct substrate specificity of protein farnesyltransferase from Trypanosoma brucei. J. Biol. Chem. 275, 21870–21876 (2000).
Eastman, R.T., Buckner, F.S., Yokoyama, K., Gelb, M.H. & Van Voorhis, W.C. Thematic review series: lipid posttranslational modifications. Fighting parasitic disease by blocking protein farnesylation. J. Lipid Res. 47, 233–240 (2006).
Buckner, F.S., Eastman, R.T., Yokoyama, K., Gelb, M.H. & Van Voorhis, W.C. Protein farnesyl transferase inhibitors for the treatment of malaria and African trypanosomiasis. Curr. Opin. Investig. Drugs 6, 791–797 (2005).
Gelb, M.H. et al. Protein farnesyl and N-myristoyl transferases: piggy-back medicinal chemistry targets for the development of antitrypanosomatid and antimalarial therapeutics. Mol. Biochem. Parasitol. 126, 155–163 (2003).
Eastman, R.T. et al. Resistance to a protein farnesyltransferase inhibitor in Plasmodium falciparum. J. Biol. Chem. 280, 13554–13559 (2005).
Nallan, L. et al. Protein farnesyltransferase inhibitors exhibit potent antimalarial activity. J. Med. Chem. 48, 3704–3713 (2005).
Acknowledgements
This work was supported by US National Institutes of Health grants AI054384 to M.H.G., GM41223 to S.M. and CA32737 and CA41996 to F.T.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Gelb, M., Brunsveld, L., Hrycyna, C. et al. Therapeutic intervention based on protein prenylation and associated modifications. Nat Chem Biol 2, 518–528 (2006). https://doi.org/10.1038/nchembio818
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio818
This article is cited by
-
Protein prenylation: unique fats make their mark on biology
Nature Reviews Molecular Cell Biology (2016)
-
Aβ-Induced Insulin Resistance and the Effects of Insulin on the Cholesterol Synthesis Pathway and Aβ Secretion in Neural Cells
Neuroscience Bulletin (2016)
-
Nerve growth factor induces neurite outgrowth of PC12 cells by promoting Gβγ-microtubule interaction
BMC Neuroscience (2014)
-
Statins and Neuroprotection: Basic Pharmacology Needed
Molecular Neurobiology (2014)
-
In vitro and in vivo effects of geranylgeranyltransferase I inhibitor P61A6 on non-small cell lung cancer cells
BMC Cancer (2013)