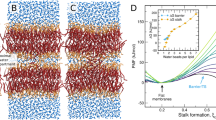
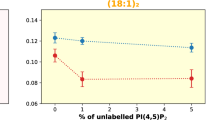
Abstract
Trafficking and sorting of membrane-anchored Ras GTPases are regulated by partitioning between distinct membrane domains. Here, in vitro experiments and microscopic molecular theory reveal membrane curvature as a new modulator of N-Ras lipid anchor and palmitoyl chain partitioning. Membrane curvature was essential for enrichment in raft-like liquid-ordered phases; enrichment was driven by relief of lateral pressure upon anchor insertion and most likely affects the localization of lipidated proteins in general.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cox, A.D. & Der, C. Small GTPases 1, 2–27 (2010).
Hancock, J.F. Nat. Rev. Mol. Cell Biol. 4, 373–384 (2003).
Belanis, L., Plowman, S.J., Rotblat, B., Hancock, J.F. & Kloog, Y. Mol. Biol. Cell 19, 1404–1414 (2008).
Hancock, J.F. & Parton, R.G. Biochem. J. 389, 1–11 (2005).
Prior, I.A. et al. Nat. Cell Biol. 3, 368–375 (2001).
Yan, J., Roy, S., Apolloni, A., Lane, A. & Hancock, J.F. J. Biol. Chem. 273, 24052–24056 (1998).
Weise, K., Triola, G., Brunsveld, L., Waldmann, H. & Winter, R. J. Am. Chem. Soc. 131, 1557–1564 (2009).
Johnson, S.A. et al. Biochim. Biophys. Acta 1798, 1427–1435 (2010).
Nicolini, C. et al. J. Am. Chem. Soc. 128, 192–201 (2006).
Simons, K. & Gerl, M.J. Nat. Rev. Mol. Cell Biol. 11, 688–699 (2010).
Anderson, R.G.W. Annu. Rev. Biochem. 67, 199–225 (1998).
Kunding, A.H., Mortensen, M.W., Christensen, S.M. & Stamou, D. Biophys. J. 95, 1176–1188 (2008).
Bendix, P.M., Pedersen, M.S. & Stamou, D. Proc. Natl. Acad. Sci. USA 106, 12341–12346 (2009).
Hatzakis, N.S. et al. Nat. Chem. Biol. 5, 835–841 (2009).
Kamal, M.M., Mills, D., Grzybek, M. & Howard, J. Proc. Natl. Acad. Sci. USA 106, 22245–22250 (2009).
Roux, A. et al. EMBO J. 24, 1537–1545 (2005).
Vamparys, L. et al. Biophys. J. 104, 585–593 (2013).
Meinhardt, S., Vink, R.L.C. & Schmid, F. Proc. Natl. Acad. Sci. USA 110, 4476–4481 (2013).
Cui, H., Lyman, E. & Voth, G.A. Biophys. J. 100, 1271–1279 (2011).
Uline, M.J., Longo, G.S., Schick, M. & Szleifer, I. Biophys. J. 98, 1883–1892 (2010).
Sampaio, J.L., Moreno, M.J. & Vaz, W.L.C. Biophys. J. 88, 4064–4071 (2005).
Abreu, M.S.C., Moreno, M.J. & Vaz, W.L.C. Biophys. J. 87, 353–365 (2004).
Sezgin, E. et al. Biochim. Biophys. Acta 1818, 1777–1784 (2012).
Parthasarathy, R., Yu, C.H. & Groves, J.T. Langmuir 22, 5095–5099 (2006).
Baumgart, T., Hess, S.T. & Webb, W.W. Nature 425, 821–824 (2003).
Shahinian, S. & Silvius, J.R. Biochemistry 34, 3813–3822 (1995).
Chiu, V.K. et al. J. Biol. Chem. 279, 7346–7352 (2004).
Hughes, L.D., Rawle, R.J. & Boxer, S.G. PLoS ONE 9, e87649 (2014).
Veatch, S.L. & Keller, S.L. Biochim. Biophys. Acta. 1746, 172–185 (2005).
Bhatia, V.K. et al. EMBO J. 28, 3303–3314 (2009).
Bhatia, V.K., Hatzakis, N.S. & Stamou, D. Semin. Cell Dev. Biol. 21, 381–390 (2010).
Rocks, O. et al. Science 307, 1746–1752 (2005).
Larsen, J., Hatzakis, N.S. & Stamou, D. J. Am. Chem. Soc. 133, 10685–10687 (2011).
Elizondo, E. et al. J. Am. Chem. Soc. 134, 1918–1921 (2012).
Silvius, J.R. Biochim. Biophys. Acta. 1746, 193–202 (2005).
Acknowledgements
This work was supported by the Lundbeck Foundation Center for Biomembranes in Nanomedicine, the Danish Councils for Independent and Strategic Research and the University of Copenhagen programs of excellence, 'Single-Molecule Nanoscience', 'BioScaRT' and 'UNIK-Synthetic Biology'. I.S. would like to acknowledge support from the U.S. National Science Foundation under grant no. CBET-1403058, and M.J.U acknowledges support from the US National Institutes of Health under grant no. P20GM103499.
Author information
Authors and Affiliations
Contributions
D.S. conceived the strategy and was responsible for the overall project management. D.S., N.S.H., J.B.L. and M.B.J designed all experiments, which were performed by J.B.L. and M.B.J with help from N.S.H. and V.K.B. S.L.P. and K.J.J. synthesized and purified tN-Ras. M.J.U. and I.S. performed theoretical calculations of anchor partitioning. J.B.L., D.S., L.I. and N.S.H. wrote the manuscript. D.S. and N.S.H. supervised the project. All authors discussed the results and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results and Supplementary Figures 1–14. (PDF 4117 kb)
Supplementary Note
Supplementary Note (PDF 781 kb)
Rights and permissions
About this article
Cite this article
Larsen, J., Jensen, M., Bhatia, V. et al. Membrane curvature enables N-Ras lipid anchor sorting to liquid-ordered membrane phases. Nat Chem Biol 11, 192–194 (2015). https://doi.org/10.1038/nchembio.1733
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1733
This article is cited by
-
Molecular mechanism of GPCR spatial organization at the plasma membrane
Nature Chemical Biology (2023)
-
TGFβ-induced changes in membrane curvature influence Ras oncoprotein membrane localization
Scientific Reports (2022)
-
Enhanced CHOLESTEROL biosynthesis promotes breast cancer metastasis via modulating CCDC25 expression and neutrophil extracellular traps formation
Scientific Reports (2022)
-
Remodeling of the Plasma Membrane by Surface-Bound Protein Monomers and Oligomers: The Critical Role of Intrinsically Disordered Regions
The Journal of Membrane Biology (2022)
-
Pattern formation in reaction–diffusion system on membrane with mechanochemical feedback
Scientific Reports (2020)