Abstract
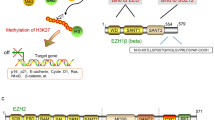
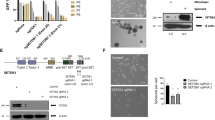
Enhancer of zeste homolog 2 (EZH2) is the histone lysine N-methyltransferase component of the Polycomb repressive complex 2 (PRC2), which, in conjunction with embryonic ectoderm development (EED) and suppressor of zeste 12 homolog, regulates cell lineage determination and homeostasis. Enzymatic hyperactivity has been linked to aberrant repression of tumor suppressor genes in diverse cancers. Here, we report the development of stabilized α-helix of EZH2 (SAH-EZH2) peptides that selectively inhibit H3 Lys27 trimethylation by dose-responsively disrupting the EZH2–EED complex and reducing EZH2 protein levels, a mechanism distinct from that reported for small-molecule EZH2 inhibitors targeting the enzyme catalytic domain. MLL-AF9 leukemia cells, which are dependent on PRC2, undergo growth arrest and monocyte-macrophage differentiation upon treatment with SAH-EZH2, consistent with observed changes in expression of PRC2-regulated, lineage-specific marker genes. Thus, by dissociating the EZH2–EED complex, we pharmacologically modulate an epigenetic 'writer' and suppress PRC2-dependent cancer cell growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cao, R. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039–1043 (2002).
Czermin, B. et al. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111, 185–196 (2002).
Müller, J. et al. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 111, 197–208 (2002).
Kuzmichev, A. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev. 16, 2893–2905 (2002).
Varambally, S. et al. The Polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419, 624–629 (2002).
Kleer, C.G. et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc. Natl. Acad. Sci. USA 100, 11606–11611 (2003).
He, L.-R. et al. High expression of EZH2 is associated with tumor aggressiveness and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Int. J. Cancer 127, 138–147 (2010).
Wang, C. et al. EZH2 mediates epigenetic silencing of neuroblastoma suppressor genes CASZ1, CLU, RUNX3, and NGFR. Cancer Res. 72, 315–324 (2012).
Yu, J. et al. Integrative genomics analysis reveals silencing of β-adrenergic signaling by Polycomb in prostate cancer. Cancer Cell 12, 419–431 (2007).
Kong, D. et al. Loss of let-7 up-regulates EZH2 in prostate cancer consistent with the acquisition of cancer stem cell signatures that are attenuated by BR-DIM. PLoS ONE 7, e33729 (2012).
Varambally, S. et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science 322, 1695–1699 (2008).
Morin, R.D. et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat. Genet. 42, 181–185 (2010).
McCabe, M.T. et al. Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27 (H3K27). Proc. Natl. Acad. Sci. USA 109, 2989–2994 (2012).
Béguelin, W. et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 23, 677–692 (2013).
Fussbroich, B. et al. EZH2 depletion blocks the proliferation of colon cancer cells. PLoS ONE 6, e21651 (2011).
Fujii, S., Ito, K., Ito, Y. & Ochiai, A. Enhancer of zeste homologue 2 (EZH2) down-regulates RUNX3 by increasing histone H3 methylation. J. Biol. Chem. 283, 17324–17332 (2008).
Wilson, B.G. et al. Epigenetic antagonism between Polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell 18, 316–328 (2010).
Chamberlain, S.J., Yee, D. & Magnuson, T. Polycomb repressive complex 2 is dispensable for maintenance of embryonic stem cell pluripotency. Stem Cells 26, 1496–1505 (2008).
Shen, X. et al. Jumonji modulates Polycomb activity and self-renewal versus differentiation of stem cells. Cell 139, 1303–1314 (2009).
Knutson, S.K. et al. A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat. Chem. Biol. 8, 890–896 (2012).
McCabe, M.T. et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492, 108–112 (2012).
Han, Z. et al. Structural basis of EZH2 recognition by EED. Structure 15, 1306–1315 (2007).
Neff, T. et al. Polycomb repressive complex 2 is required for MLL-AF9 leukemia. Proc. Natl. Acad. Sci. USA 109, 5028–5033 (2012).
Shi, J. et al. The Polycomb complex PRC2 supports aberrant self-renewal in a mouse model of MLL-AF9;NrasG12D acute myeloid leukemia. Oncogene 10.1038/onc.2012.110 (2013).
Walensky, L.D. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science 305, 1466–1470 (2004).
Moellering, R.E. et al. Direct inhibition of the NOTCH transcription factor complex. Nature 462, 182–188 (2009).
Deshayes, S., Morris, M.C., Divita, G. & Heitz, F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell. Mol. Life Sci. 62, 1839–1849 (2005).
Shen, X. et al. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol. Cell 32, 491–502 (2008).
Ezhkova, E. et al. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 25, 485–498 (2011).
Margueron, R. et al. Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol. Cell 32, 503–518 (2008).
Byvoet, P., Shepherd, G.R., Hardin, J.M. & Noland, B.J. The distribution and turnover of labeled methyl groups in histone fractions of cultured mammalian cells. Arch. Biochem. Biophys. 148, 558–567 (1972).
Sasaki, M., Yamaguchi, J., Itatsu, K., Ikeda, H. & Nakanuma, Y. Over-expression of Polycomb group protein EZH2 relates to decreased expression of p16 INK4a in cholangiocarcinogenesis in hepatolithiasis. J. Pathol. 215, 175–183 (2008).
Bracken, A.P. et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 21, 525–530 (2007).
Krivtsov, A.V. et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature 442, 818–822 (2006).
Toren, A. et al. CD133-positive hematopoietic stem cell 'stemness' genes contain many genes mutated or abnormally expressed in leukemia. Stem Cells 23, 1142–1153 (2005).
Suetsugu, A. et al. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem. Biophys. Res. Commun. 351, 820–824 (2006).
Singh, S.K. et al. Identification of human brain tumour initiating cells. Nature 432, 396–401 (2004).
Di Tullio, A., Vu Manh, T.P., Schubert, A., Månsson, R. & Graf, T. CCAAT/enhancer binding protein α (C/EBPα)-induced transdifferentiation of pre-B cells into macrophages involves no overt retrodifferentiation. Proc. Natl. Acad. Sci. USA 108, 17016–17021 (2011); erratum 109, 11053.
Pinto do, O.P. Hematopoietic progenitor/stem cells immortalized by Lhx2 generate functional hematopoietic cells in vivo. Blood 99, 3939–3946 (2002).
Lee, S.T. et al. Context-specific regulation of NF-κB target gene expression by EZH2 in breast cancers. Mol. Cell 43, 798–810 (2011).
Xu, K. et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science 338, 1465–1469 (2012).
You, J.S. & Jones, P.A. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell 22, 9–20 (2012).
Hinz, S. et al. Expression of the Polycomb group protein EZH2 and its relation to outcome in patients with urothelial carcinoma of the bladder. J. Cancer Res. Clin. Oncol. 134, 331–336 (2008).
Tan, J. et al. Pharmacologic disruption of Polycomb-repressive complex 2–mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 21, 1050–1063 (2007).
Momparler, R.L., Idaghdour, Y., Marquez, V.E. & Momparler, L.F. Synergistic antileukemic action of a combination of inhibitors of DNA methylation and histone methylation. Leuk. Res. 36, 1049–1054 (2012).
Miranda, T.B. et al. DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation. Mol. Cancer Ther. 8, 1579–1588 (2009).
Chiang, P.K. Biological effects of inhibitors of S-adenosylhomocysteine hydrolase. Pharmacol. Ther. 77, 115–134 (1998).
Borchardt, R.T., Keller, B.T. & Patel-Thombre, U. Neplanocin A. A potent inhibitor of S-adenosylhomocysteine hydrolase and of vaccinia virus multiplication in mouse L929 cells. J. Biol. Chem. 259, 4353–4358 (1984).
Bowen, N.J., Fujita, N., Kajita, M. & Wade, P.A. Mi-2/NuRD: multiple complexes for many purposes. Biochim. Biophys. Acta 1677, 52–57 (2004).
Ho, L. & Crabtree, G.R. Chromatin remodelling during development. Nature 463, 474–484 (2010).
Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 58, 621–681 (2006).
Walensky, L.D. et al. A stapled BID BH3 helix directly binds and activates BAX. Mol. Cell 24, 199–210 (2006).
Stewart, M.L., Fire, E., Keating, A.E. & Walensky, L.D. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat. Chem. Biol. 6, 595–601 (2010).
Bird, G.H. et al. Hydrocarbon double-stapling remedies the proteolytic instability of a lengthy peptide therapeutic. Proc. Natl. Acad. Sci. USA 107, 14093–14098 (2010).
Acknowledgements
We thank E. Smith for graphics assistance. This work was supported by US National Institutes of Health grant U01CA105423 to S.H.O. and 5R01GM090299 and a Leukemia and Lymphoma Society Specialized Center of Research project grant to L.D.W. S.H.O. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
W.K., L.D.W. and S.H.O. designed the experiments and wrote the manuscript. W.K. and G.H.B. synthesized the stapled peptides. T.N. generated MLL-AF9 cells and assisted W.K. with the colony-forming assay and morphological studies. G.G. performed the high-throughput microfluidic qPCR. M.A.K. assisted W.K. with the studies on HPC5, M1 and C1498 cell lines.
Corresponding authors
Ethics declarations
Competing interests
L.D.W. is a scientific advisory board member and consultant for Aileron Therapeutics.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–21 and Supplementary Tables 1 and 2. (PDF 9479 kb)
Rights and permissions
About this article
Cite this article
Kim, W., Bird, G., Neff, T. et al. Targeted disruption of the EZH2–EED complex inhibits EZH2-dependent cancer. Nat Chem Biol 9, 643–650 (2013). https://doi.org/10.1038/nchembio.1331
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1331
This article is cited by
-
EZH2 K63-polyubiquitination affecting migration in extranodal natural killer/T-cell lymphoma
Clinical Epigenetics (2023)
-
Phosphorylation and stabilization of EZH2 by DCAF1/VprBP trigger aberrant gene silencing in colon cancer
Nature Communications (2023)
-
Dual inhibition of EZH1/2 induces cell cycle arrest of B cell acute lymphoblastic leukemia cells through upregulation of CDKN1C and TP53INP1
International Journal of Hematology (2023)
-
SUZ12 inhibition attenuates cell proliferation of glioblastoma via post-translational regulation of CDKN1B
Genes & Genomics (2023)
-
The long and short non-coding RNAs modulating EZH2 signaling in cancer
Journal of Hematology & Oncology (2022)