Abstract
The zinc-finger transcription factor Snail1 is inappropriately expressed in breast cancer and associated with poor prognosis. While interrogating human databases, we uncovered marked decreases in relapse-free survival of breast cancer patients expressing high Snail1 levels in tandem with wild-type, but not mutant, p53. Using a Snail1 conditional knockout model of mouse breast cancer that maintains wild-type p53, we find that Snail1 plays an essential role in tumour progression by controlling the expansion and activity of tumour-initiating cells in preneoplastic glands and established tumours, whereas it is not required for normal mammary development. Growth and survival of preneoplastic as well as neoplastic mammary epithelial cells is dependent on the formation of a Snail1/HDAC1/p53 tri-molecular complex that deacetylates active p53, thereby promoting its proteasomal degradation. Our findings identify Snail1 as a molecular bypass that suppresses the anti-proliferative and pro-apoptotic effects exerted by wild-type p53 in breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Collado, M. & Serrano, M. Senescence in tumours: evidence from mice and humans. Nat. Rev. Cancer 10, 51–57 (2010).
Puisieux, A., Brabletz, T. & Caramel, J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 16, 488–494 (2014).
Olivier, M., Hollstein, M. & Hainaut, P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2, a001008 (2010).
Moody, S. E. et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 8, 197–209 (2005).
Yook, J. I. et al. A Wnt-Axin2-GSK3β cascade regulates Snail1 activity in breast cancer cells. Nat. Cell Biol. 8, 1398–1406 (2006).
Olmeda, D. et al. SNAI1 is required for tumor growth and lymph node metastasis of human breast carcinoma MDA-MB-231 cells. Cancer Res. 67, 11721–11731 (2007).
Zhang, K. et al. The collagen receptor discoidin domain receptor 2 stabilizes Snail1 to facilitate breast cancer metastasis. Nat. Cell Biol. 15, 677–687 (2013).
Tran, H. D. et al. Transient Snail1 expression is necessary for metastatic competence in breast cancer. Cancer Res. 74, 6330–6340 (2014).
Ye, X. et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 525, 256–260 (2015).
Mani, S. A. et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Nieto, M. A. Epithelial plasticity: a common theme in embryonic and cancer cells. Science 342, 1234850 (2013).
Tam, W. L. & Weinberg, R. A. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 19, 1438–1449 (2013).
Gyorffy, B. et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res. Treat. 123, 725–731 (2010).
Gyorffy, B. et al. TP53 mutation-correlated genes predict the risk of tumor relapse and identify MPS1 as a potential therapeutic kinase in TP53-mutated breast cancers. Mol. Oncol. 8, 508–519 (2014).
Kajita, M., McClinic, K. N. & Wade, P. A. Aberrant expression of the transcription factors Snail and Slug alters the response to genotoxic stress. Mol. Cell Biol. 24, 7559–7566 (2004).
Escriva, M. et al. Repression of PTEN phosphatase by Snail1 transcriptional factor during γ radiation-induced apoptosis. Mol. Cell Biol. 28, 1528–1540 (2008).
Lee, S. H. et al. Blocking of p53-Snail binding, promoted by oncogenic K-Ras, recovers p53 expression and function. Neoplasia 11, 22–31 (2009).
Lim, S. O., Kim, H. & Jung, G. p53 inhibits tumor cell invasion via the degradation of Snail protein in hepatocellular carcinoma. FEBS Lett. 584, 2231–2236 (2010).
Perez-Mancera, P. A. et al. Cancer development induced by graded expression of Snail in mice. Hum. Mol. Genet. 14, 3449–3461 (2005).
Rowe, R. G. et al. Mesenchymal cells reactivate Snail1 expression to drive three-dimensional invasion programs. J. Cell Biol. 184, 399–408 (2009).
Lin, Y. et al. Snail1-dependent control of embryonic stem cell pluripotency and lineage commitment. Nat. Commun. 5, 3070 (2014).
Wu, Z. Q. et al. A Snail1/Notch1 signalling axis controls embryonic vascular development. Nat. Commun. 5, 3998 (2014).
Grande, M. T. et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat. Med. 21, 989–997 (2015).
Guy, C. T., Cardiff, R. D. & Muller, W. J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol. Cell Biol. 12, 954–961 (1992).
Lin, E. Y. et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am. J. Pathol. 163, 2113–2126 (2003).
Cheung, K. J., Gabrielson, E., Werb, Z. & Ewald, A. J. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 155, 1639–1651 (2013).
Geradts, J. et al. Nuclear Snail1 and nuclear ZEB1 protein expression in invasive and intraductal human breast carcinomas. Hum. Pathol. 42, 1125–1131 (2011).
Wu, Z. Q. et al. Canonical Wnt signaling regulates Slug activity and links epithelial-mesenchymal transition with epigenetic Breast Cancer 1, Early Onset (BRCA1) repression. Proc. Natl Acad. Sci. USA 109, 16654–16659 (2012).
Muenst, S. et al. Nuclear expression of Snail is an independent negative prognostic factor in human breast cancer. Dis. Markers 35, 337–344 (2013).
Wagner, K. U. et al. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res. 10, 545–553 (2001).
Jiao, B. et al. Paternal RLIM/Rnf12 is a survival factor for milk-producing alveolar cells. Cell 149, 630–641 (2012).
Chen, Q. et al. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes Dev. 28, 432–437 (2014).
Shackleton, M. et al. Generation of a functional mammary gland from a single stem cell. Nature 439, 84–88 (2006).
Asselin-Labat, M. L. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat. Cell Biol. 9, 201–209 (2007).
Kouros-Mehr, H. et al. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell 13, 141–152 (2008).
Wan, L. et al. MTDH-SND1 interaction is crucial for expansion and activity of tumor-initiating cells in diverse oncogene- and carcinogen-induced mammary tumors. Cancer Cell 26, 92–105 (2014).
Malanchi, I. et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89 (2011).
Thiery, J. P., Acloque, H., Huang, R. Y. & Nieto, M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Dong, C. et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell 23, 316–331 (2013).
Ota, I., Li, X. Y., Hu, Y. & Weiss, S. J. Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proc. Natl Acad. Sci. USA 106, 20318–20323 (2009).
Ansieau, S. et al. Induction of EMT by Twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 14, 79–89 (2008).
Pyun, B. J. et al. Mutual regulation between DNA-PKcs and Snail1 leads to increased genomic instability and aggressive tumor characteristics. Cell Death Dis. 4, e517 (2013).
Kang, G. Y. et al. Inhibition of Snail1-DNA-PKcs protein–protein interface sensitizes cancer cells and inhibits tumor metastasis. J. Biol. Chem. 288, 32506–32516 (2013).
Tang, Y., Zhao, W., Chen, Y., Zhao, Y. & Gu, W. Acetylation is indispensable for p53 activation. Cell 133, 612–626 (2008).
Thompson, P. R. et al. Regulation of the p300 HAT domain via a novel activation loop. Nat. Struct. Mol. Biol. 11, 308–315 (2004).
Ferreon, J. C. et al. Cooperative regulation of p53 by modulation of ternary complex formation with CBP/p300 and HDM2. Proc. Natl Acad. Sci. USA 106, 6591–6596 (2009).
Villarejo, A., Cortes-Cabrera, A., Molina-Ortiz, P., Portillo, F. & Cano, A. Differential role of Snail1 and Snail2 zinc fingers in E-cadherin repression and epithelial to mesenchymal transition. J. Biol. Chem. 289, 930–941 (2014).
Peinado, H., Ballestar, E., Esteller, M. & Cano, A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol. Cell Biol. 24, 306–319 (2004).
Choi, S. et al. Structural basis for the selective nuclear import of the C2H2 zinc-finger protein Snail by importin beta. Acta Crystallogr. D Biol. Crystallogr. 70, 1050–1060 (2014).
Vincent, T. et al. A Snail1-SMAD3/4 transcriptional repressor complex promotes TGF-β mediated epithelial-mesenchymal transition. Nat. Cell Biol. 11, 943–950 (2009).
Blanco, M. J. et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene 21, 3241–3246 (2002).
Trimboli, A. J. et al. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res. 68, 937–945 (2008).
Lan, L. et al. Shp2 signaling suppresses senescence in PyMT-induced mammary gland cancer in mice. EMBO J. 34, 1493–1508 (2015).
Horvay, K. et al. Snai1 regulates cell lineage allocation and stem cell maintenance in the mouse intestinal epithelium. EMBO J. 34, 1319–1335 (2015).
Wang, D. et al. Identification of multipotent mammary stem cells by protein C receptor expression. Nature 517, 81–84 (2015).
Kim, J. et al. Cooperative actions of p21WAF1 and p53 induce Slug protein degradation and suppress cell invasion. EMBO Rep. 15, 1062–1068 (2014).
Piccinin, S. et al. A ‘Twist box’ code of p53 inactivation: Twist box: p53 interaction promotes p53 degradation. Cancer Cell 22, 404–415 (2012).
Shiota, M. et al. Twist and p53 reciprocally regulate target genes via direct interaction. Oncogene 27, 5543–5553 (2008).
Beck, B. et al. Different levels of Twist1 regulate skin tumor initiation, stemness, and progression. Cell Stem Cell 16, 67–79 (2015).
Gajula, R. P. et al. The Twist box domain is required for Twist1-induced prostate cancer metastasis. Mol. Cancer Res. 11, 1387–1400 (2013).
Wang, S. P. et al. p53 controls cancer cell invasion by inducing the MDM2-mediated degradation of Slug. Nat. Cell Biol. 11, 694–704 (2009).
Lamouille, S., Xu, J. & Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196 (2014).
Hsu, D. S. et al. Acetylation of Snail modulates the cytokinome of cancer cells to enhance the recruitment of macrophages. Cancer Cell 26, 534–548 (2014).
Ito, A. et al. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J. 21, 6236–6245 (2002).
Gadea, G., de Toledo, M., Anguille, C. & Roux, P. Loss of p53 promotes RhoA-ROCK-dependent cell migration and invasion in 3D matrices. J. Cell Biol. 178, 23–30 (2007).
Godar, S. et al. Growth-inhibitory and tumor-suppressive functions of p53 depend on its repression of CD44 expression. Cell 134, 62–73 (2008).
Mak, A. S. p53 in cell invasion, podosomes, and invadopodia. Cell Adhes. Migr. 8, 205–214 (2014).
Chang, C. J. et al. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat. Cell Biol. 13, 317–323 (2011).
Fischer, K. R. et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527, 472–476 (2015).
Zheng, X. et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527, 525–530 (2015).
Acknowledgements
This research was supported by a grant from the Breast Cancer Research Foundation (BCRF) to S.J.W. as well as the Startup Fund from China Pharmaceutical University, the National Natural Science Foundation of China (NSFC 81572745 and 91539115), and the Jiangsu Province Innovative Research Team and State Key Laboratory of Natural Medicines (SKLNMZZJQ201604) to Z.-Q.W. Additional support was provided by the Changjiang Scholar and Innovative Research Team (IRT1193) to Q.-L.G. and the US National Institutes of Health (U01 CA180980) to X.S.L. We thank M.-D. Lai (China Pharmaceutical University, China) for critically reviewing this manuscript, W. Gu (Columbia University, USA) and P.-C. Yang (Academia Sinica, Taiwan) for providing HA-tagged p53 WT and mutant plasmids, R. Weinberg (Massachusetts Institute of Technology, USA) for sharing human mammary epithelial cells (HMLEs), A. Cano (Universidad Autonoma de Madrid, Spain) for Snail1 mutants, Y. Kang and L. Wan (Princeton University, USA) for sharing protocols of mouse mammary epithelial cell isolation, B. Gyorffy (Hungarian Academy of Sciences, Hungary) for assistance with the human breast cancer databases and R. Kuick (University of Michigan, USA) for helpful discussions.
Author information
Authors and Affiliations
Contributions
Z.-Q.W. and S.J.W. designed and supervised the project, analysed data, wrote the manuscript and approved the final version; T.N., X.-Y.L. and N.L. conducted experiments and analysed data. T.A., R.F., W.-C.L., Y.-W.Z., R.G.R., Y.-S.L., A.S. and T.F. conducted experiments. Z.-P.L., X.-J.X., X.-Q.Z., B.-A.C., X.S.L. and Q.-L.G. analysed data and provided relevant advice.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
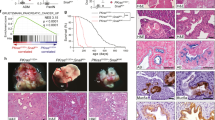
Supplementary Figure 1 A relationship between relapse-free survival rate and Snail1 expression level in different molecular subtypes of breast cancer patients.
(a,b) Kaplan–Meier survival analysis of the relationship between relapse-free survival (RFS) rates and Snail1 expression level in HER2+ (a) or basal (b) subtype of breast cancer patients bearing wild-type or mutant TP53 alleles. Survival data are fitted by the ‘survfit’ function. Kaplan-Meier curves are drawn by the ‘ggsurv’ function in R package ‘survival’. Differences between two survival curves are measured by the G-rho family of tests. n represents number of patients.
Supplementary Figure 2 Snail1 excision in MaECs impairs PyMT-induced tumour progression without affecting mammary gland development or lactation.
(a) Cross-sections of X-gal/LacZ staining in 7 wk-old preneoplastic glands (left panel) and 19 wk-old primary tumours (right panel) derived from PyMT-cKO;ROSA-LacZ mice (the image is representative of images from 5 mice). Strong X-gal/LacZ staining is detected in the epithelium, but not stromal compartment. (b) Western blot analysis of Lin−pNECs or Lin−NECs derived from preneoplastic glands (7 wks of age) and neoplastic tumours (19 wks of age) of PyMT-WT and PyMT-cKO;ROSA-LacZ mice (results are representatives of five independent experiments). (c) Gross view of primary tumours isolated from 15 wk-old PyMT-WT and PyMT-cKO mice (the image is representative of images from 5 mice). Dashed areas outline the primary tumours developing from the proximal to distal sites of the mammary glands. (d) Whole-mount Carmine red staining of mammary glands from WT and cKO mice at 5, 9 and 15 weeks of age as well as lactation day 3 (the image is representative of images from 5 mice). Unprocessed blots are shown in Supplementary Fig. 8.
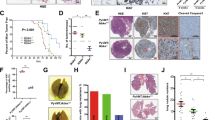
Supplementary Figure 3 Flow cytometry analysis of preneoplastic glands from PyMT-WT and -cKO mice.
(a) Quantification of Lin−CD29loCD24+ basal subpopulation in normal glands from WT and cKO mice as well as preneoplastic glands from PyMT-WT and-cKO mice. Data are presented as mean ± s.e.m. (n = 6 independent experiments). ∗∗P < 0.01, two-sided Student’s t test. (b) Flow cytometry of Lin−CD29+CD90+ subpopulation in preneoplastic glands from PyMT-WT and-cKO mice (the image is representative of images from 6 mice). (c) Quantification of Lin−CD29+CD90+ subpopulation in preneoplastic glands as described in (b). Data are presented as mean ± s.e.m. (n = 6 independent experiments). N.S., not significant, two-sided Student’s t test. (d) Size of tumourspheres formed by PyMT-WT or-cKO pNECs dissected from mammary glands of 6 wk-old mice. 1°, 2° and 3° denote the three successive generations of pNECs used in the assay, respectively. Data are presented as mean ± s.e.m. (n = 6 independent experiments). ∗∗P < 0.01, two-sided Student’s t test.
Supplementary Figure 4 Preneoplastic glands and advanced tumours from PyMT-cKO mice exhibit defects in collective invasion, proliferation and survival.
(a) Immunohistochemical staining of K14 expression at the tumour-stromal borders in primary tumours isolated from 13 wk-old PyMT-WT and-cKO mice (the image is representative of images from 5 mice). Magnified images of boxed areas in red and brown (at left) are shown in the middle and right panels, respectively. Arrows mark invasive strands that are characteristic of K14+ invading leader cells at the tumour-stromal borders. (b) Immunohistochemical staining of Ki67, phospho-Histone H3 and cleaved caspase 3 in the preneoplastic mammary glands of 4 wk-old PyMT-WT and-cKO females (the image is representative of images from 5 mice). Magnified areas of boxed sections are shown in the bottom panels. Green arrows in middle panels denote phospho-Histone H3-positive cells (that is, proliferative cells). Red arrows in right panels denote cleaved caspase 3-positive cells (that is, apoptotic cells). (c–e) Quantification of Ki67 (c), phospho-Histone H3 (p-H3; d) and cleaved caspase 3 (C-casp3; e) positive cells (%) as shown in (b). One thousand to two thousand cells were counted in 10 random fields of each slide. Data are presented as mean ± s.e.m. (n = 5 independent experiments). ∗P < 0.05, ∗∗P < 0.01, two-sided Student’s t test.
Supplementary Figure 5 Snail1 maintains TIC function in established adenocarcinomas.
(a) Flow cytometry analysis (CD24/CD29/CD61 profiling) of LIN− mammary epithelial cells isolated from transplanted tumours (n = 5, each) that were derived from adeno-βGal or-Cre transduced Snail1fl/fl adenocarcinoma cells. Tumours were retrieved 9 wks post-transduction. (b) Quantification of LIN−CD29loCD24+/LIN−CD29loCD61+ subpopulations in control and Snail1-deleted orthotopic tumours. Data are presented as mean ± s.e.m. (n = 5 independent experiments). NS, not significant, two-sided Student’s t test. (c) Flow cytometry analysis (CD24/CD90 profiling) of LIN− mammary epithelial cells isolated from transplanted tumours (the image is representative of images from 5 mice) as described in (a). (d) Quantification of LIN−CD24+CD90+ subpopulations in control and Snail1-deleted orthotopic tumours. Data are presented as mean ± s.e.m. (n = 5 independent experiments). NS, not significant, two-sided Student’s t test. (e) Flow cytometry analysis of ALDH activity of LIN− mammary epithelial cells isolated from transplanted tumours (the image is representative of images from 5 tumours) that were derived from Snail1fl/fl adenocarcinoma cells. Tumours were retrieved 9 wks post-transduction. DEAB-treated samples were used as gating control (left panel). (f,g) Sorted Lin−ALDH− and Lin−ALDH+ cells from transplanted tumours as described in (e) were subjected to tumoursphere formation assay. Representative tumourspheres are shown (f) with quantification of tumourshphere formation (number and size) (g). Data are presented as mean ± s.e.m. (n = 5 independent experiments). ∗∗P < 0.01, two-sided Student’s t test. (h) Flow cytometry analysis (CD24/CD61/CD29/ALDH profiling) of Lin− mammary epithelial cells isolated from transplanted tumours (the image is representative of images from 5 tumorus) as described in (e). (i) Quantification of Lin−ALDH+ TICs isolated from control and Snail1-deleted orthotopic tumours. Data are presented as mean ± s.e.m. (n = 5 independent experiments). ∗P < 0.05, two-sided Student’s t test. (j) Quantification of tumoursphere formation (number and size) by adeno-βGal or-Cre transduced Lin−ALDH+ cells sorted from transplanted tumours as described in (e). Data are presented as mean ± s.e.m. (n = 5 independent experiments). ∗P < 0.05, ∗∗P < 0.01, two-sided Student’s t test. Source data are provided in Supplementary Table 1 (h).
Supplementary Figure 6 Deletion of Snail1 represses tumour proliferation and survival but does not affect Rb activity or induce DNA damage in PyMT-induced tumours.
(a) Immunohistochemical staining of Ki67, phospho-Histone H3 and cleaved caspase 3 in control and Snail1-deleted orthotopic tumours (the image is representative of images from 5 tumours). Red arrows in panels to the right indicate cleaved caspase 3-positive cells. (b) Quantification of Ki67, phospho-Histone H3 (p-H3) and cleaved caspase 3 (C-casp3) positive cells (%) as shown in (a). One thousand to two thousand cells were counted in 10 random fields of each slide. Data are presented as mean ± s.e.m. (n = 5 independent experiments). ∗∗P < 0.01, two-sided Student’s t test. (c-e) Immunohistochemical staining of p-Rb (c), p-ATM (d) and γ-H2AX (e) in preneoplastic glands of 7 wk-old PyMT-WT or PyMT-cKO mice (left panels) or formed from neoplastic Snail1fl/fl mammary epithelial cells that were transduced with adeno-βGal or-Cre prior to a 9 wk transplantation period (right panels). The image is representative of images from 5 mice or orthotopic tumours. (f) Quantification of p-Rb, p-ATM and γ-H2AX positive cells (%) as shown in c–e. One thousand to two thousand cells were counted in 10 random fields of each slide. Data are presented as mean ± s.e.m. (n = 5 independent experiments). NS, not significant, two-sided Student’s t test. (g) Immunoblot analysis of Lin−NECs isolated from primary tumours of 12 wk-old PyMT-WT and PyMT-cKO mice (the image is representative of images from 5 mice). Arrows denote the specific bands with their expected molecular weights. Unprocessed blots are shown in Supplementary Fig. 8.
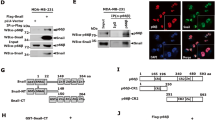
Supplementary Figure 7 Snail1 binds and negatively regulates p53 protein levels.
(a) pNECs or NECs were recovered from 7 wk-old or 19 wk-old PyMT-WT mice, respectively. Cells were transduced with adeno-βGal or-Cre, and subjected to RT-PCR analysis. Data are presented as mean ± s.e.m. (n = 5 independent experiments). ∗∗P < 0.01, one-way ANOVA test. (b) Cycloheximide (CHX; 100 μg ml−1) pulse-chase analysis of p53 protein levels in control or Snail1-deleted pNECs as shown in a. Quantification of p53 protein expression levels are shown in bottom panel. (c) 293T cells were co-transfected with the indicated plasmids at a ratio of 1:10 and treated with MG132 (10 μM) for 6 h. Cell lysates collected for IP) analysis. (d) Immunofluorescent staining of p53 and Pan-AcK in NECs as described in (c). Arrows denote the cells expressing comparable levels of p53 between adeno-βGal and-Cre transduced cells. (e) GST control or GST-p53 protein bound to GST beads was co-incubated with FLAG-tagged Snail1 protein purified from rabbit reticulocyte lysates, and the mixture subjected to GST pull-down assays. (f) Lysates form MDA-MB-231 cells were subjected to IP assay. (g) 293T cells were co-transfected with equal amount of HA-p53-WT and FLAG-Snail1, and the cell lysates were prepared and subjected to IP analysis. (h) 293T cells co-transfected with GFP-p53 and FLAG-Snail1 at a ratio of 1:10, and then treated with 1 μM trichostatin A (TSA) for 24 h. Cell lysates were prepared for immunoblotting. (i) 293T cells were co-transfected with the indicated plasmids at a ratio of 1:1:10 and treated with DMSO or MG132 (10 μM) for 6 h. Cell lysates collected for IP analysis. The numbers shown in the blots are the ratios of MDM2/p53 in the IP’ed products. Blots are representatives of three independent experiments. Asterisks and arrows denote IgGs and specific bands with their expected molecular weights, respectively. Results are representatives of three (c,e–i) or five (b,d) independent experiments. Unprocessed blots are shown in Supplementary Fig. 8. Source data are provided in Supplementary Table 1 (b,i).
Supplementary information
Supplementary Information
Supplementary Information (PDF 2057 kb)
Supplementary Table 1
Supplementary Information (XLSX 57 kb)
Rights and permissions
About this article
Cite this article
Ni, T., Li, XY., Lu, N. et al. Snail1-dependent p53 repression regulates expansion and activity of tumour-initiating cells in breast cancer. Nat Cell Biol 18, 1221–1232 (2016). https://doi.org/10.1038/ncb3425
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3425
This article is cited by
-
p52-ZER6: a determinant of tumor cell sensitivity to MDM2-p53 binding inhibitors
Acta Pharmacologica Sinica (2023)
-
Intestinal epithelial SNAI1 promotes the occurrence of colorectal cancer by enhancing EMT and Wnt/β-catenin signaling
Medical Oncology (2023)
-
Non-canonical functions of SNAIL drive context-specific cancer progression
Nature Communications (2023)
-
Omeprazole suppresses aggressive cancer growth and metastasis in mice through promoting Snail degradation
Acta Pharmacologica Sinica (2022)
-
A non-catalytic scaffolding activity of hexokinase 2 contributes to EMT and metastasis
Nature Communications (2022)