Abstract
Recognition of injured mitochondria for degradation by macroautophagy is essential for cellular health, but the mechanisms remain poorly understood. Cardiolipin is an inner mitochondrial membrane phospholipid. We found that rotenone, staurosporine, 6-hydroxydopamine and other pro-mitophagy stimuli caused externalization of cardiolipin to the mitochondrial surface in primary cortical neurons and SH-SY5Y cells. RNAi knockdown of cardiolipin synthase or of phospholipid scramblase-3, which transports cardiolipin to the outer mitochondrial membrane, decreased the delivery of mitochondria to autophagosomes. Furthermore, we found that the autophagy protein microtubule-associated-protein-1 light chain 3 (LC3), which mediates both autophagosome formation and cargo recognition, contains cardiolipin-binding sites important for the engulfment of mitochondria by the autophagic system. Mutation of LC3 residues predicted as cardiolipin-interaction sites by computational modelling inhibited its participation in mitophagy. These data indicate that redistribution of cardiolipin serves as an ‘eat-me’ signal for the elimination of damaged mitochondria from neuronal cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kim, I. & Lemasters, J. J. Mitophagy selectively degrades individual damaged mitochondria after photoirradiation. Antioxid. Redox Signal. 14, 1919–1928 (2011).
Kanki, T., Wang, K., Cao, Y., Baba, M. & Klionsky, D. J. Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev. Cell 17, 98–109 (2009).
Narendra, D., Tanaka, A., Suen, D. F. & Youle, R. J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 (2008).
Liu, L. et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 14, 177–185 (2012).
Schlame, M. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J. Lipid Res. 49, 1607–1620 (2008).
Beyer, K. & Nuscher, B. Specific cardiolipin binding interferes with labeling of sulfhydryl residues in the adenosine diphosphate/adenosine triphosphate carrier protein from beef heart mitochondria. Biochemistry 35, 15784–15790 (1996).
Singh, S. B. et al. Human IRGM regulates autophagy and cell-autonomous immunity functions through mitochondria. Nat. Cell Biol. 12, 1154–1165 (2010).
Sugawara, K. et al. The crystal structure of microtubule-associated protein light chain 3, a mammalian homologue of Saccharomyces cerevisiae Atg8. Genes Cells 9, 611–618 (2004).
Miyanishi, M. et al. Identification of Tim4 as a phosphatidylserine receptor. Nature 450, 435–439 (2007).
Schon, E. A. & Przedborski, S. Mitochondria: the next (neurode)generation. Neuron 70, 1033–1053 (2011).
Zhu, J. H., Guo, F., Shelburne, J., Watkins, S. & Chu, C. T. Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol. 13, 473–481 (2003).
Wong, E. & Cuervo, A. M. Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 13, 805–811 (2010).
Barth, S., Glick, D. & Macleod, K. F. Autophagy: assays and artifacts. J. Pathol. 221, 117–124 (2010).
Tyurin, V. A. et al. Oxidative lipidomics of programmed cell death. Methods Enzymol. 442, 375–393 (2008).
Crompton, M., Barksby, E., Johnson, N. & Capano, M. Mitochondrial intermembrane junctional complexes and their involvement in cell death. Biochimie 84, 143–152 (2002).
Andree, H. A. et al. Binding of vascular anticoagulant alpha (VAC alpha) to planar phospholipid bilayers. J. Biol. Chem. 265, 4923–4928 (1990).
Dagda, R. K., Zhu, J., Kulich, S. M. & Chu, C. T. Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress: implications for Parkinson’s disease. Autophagy 4, 770–782 (2008).
Chalovich, E. M., Zhu, J. H., Caltagarone, J., Bowser, R. & Chu, C. T. Functional repression of cAMP response element in 6-hydroxydopamine-treated neuronal cells. J. Biol. Chem. 281, 17870–17881 (2006).
Liu, J. et al. Role of phospholipid scramblase 3 in the regulation of tumor necrosis factor-alpha-induced apoptosis. Biochemistry 47, 4518–4529 (2008).
Huang, Z. et al. Cardiolipin deficiency leads to decreased cardiolipin peroxidation and increased resistance of cells to apoptosis. Free Radic. Biol. Med. 44, 1935–1944 (2008).
Ji, J. et al. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat. Neurosci. 15, 1407–1413 (2012).
Huang, W. et al. Crystal structure and biochemical analyses reveal Beclin 1 as a novel membrane binding protein. Cell Res. 22, 473–489 (2012).
Sun, Y., Vashisht, A. A., Tchieu, J., Wohlschlegel, J. A. & Dreier, L. Voltage-dependent Anion Channels (VDACs) Recruit Parkin to defective mitochondria to promote mitochondrial autophagy. J. Biol. Chem. 287, 40652–40660 (2012).
Geisler, S. et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 12, 119–131 (2010).
Wirawan, E. et al. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 1, e18 (2010).
Matsuda, N. et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 189, 211–221 (2010).
Vives-Bauza, C. et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl Acad. Sci. USA 107, 378–383 (2010).
Narendra, D. P. et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8, e1000298 (2010).
Kondapalli, C. et al. PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65. Open Biol. 2, 120080 (2012).
Okatsu, K. et al. p62/SQSTM1 cooperates with Parkin for perinuclear clustering of depolarized mitochondria. Genes Cells 15, 887–900 (2010).
Johansen, T. & Lamark, T. Selective autophagy mediated by autophagic adapter proteins. Autophagy 7, 279–296 (2011).
Shvets, E., Fass, E., Scherz-Shouval, R. & Elazar, Z. The N-terminus and Phe52 residue of LC3 recruit p62/SQSTM1 into autophagosomes. J. Cell Sci. 121, 2685–2695 (2008).
Van Laar, V. S. et al. Bioenergetics of neurons inhibit the translocation response of Parkin following rapid mitochondrial depolarization. Human Mol. Genet. 20, 927–940 (2011).
Cai, Q., Zakaria, H. M., Simone, A. & Sheng, Z. H. Spatial parkin translocation and degradation of damaged mitochondria via mitophagy in live cortical neurons. Curr. Biol. 22, 545–552 (2012).
Novak, I. et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 11, 45–51 (2010).
Hanna, R. A. et al. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J. Biol. Chem. 287, 19094–19104 (2012).
Connerth, M. et al. Intramitochondrial transport of phosphatidic acid in yeast by a lipid transfer protein. Science 338, 815–818 (2012).
Borisenko, G. G. et al. Macrophage recognition of externalized phosphatidylserine and phagocytosis of apoptotic Jurkat cells–existence of a threshold. Arch. Biochem. Biophys. 413, 41–52 (2003).
Ravichandran, K. S. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J. Exp. Med. 207, 1807–1817 (2010).
Rostovtseva, T. K., Kazemi, N., Weinrich, M. & Bezrukov, S. M. Voltage gating of VDAC is regulated by nonlamellar lipids of mitochondrial membranes. J. Biol. Chem. 281, 37496–37506 (2006).
Athea, Y. et al. AMP-activated protein kinase alpha2 deficiency affects cardiac cardiolipin homeostasis and mitochondrial function. Diabetes 56, 786–794 (2007).
Onyenwoke, R. U. et al. AMPK directly inhibits NDPK through a phosphoserine switch to maintain cellular homeostasis. Mol. Biol. Cell 23, 381–389 (2012).
Schlattner, U. et al. Dual function of mitochondrial Nm23-H4 in phosphotransfer and intermembrane lipid transfer: a cardiolipin-dependent switch. J. Biol. Chem. 288, 111–121 (2013).
Verdaguer, N., Corbalan-Garcia, S., Ochoa, W. F., Fita, I. & Gomez-Fernandez, J. C. Ca(2+) bridges the C2 membrane-binding domain of protein kinase Calpha directly to phosphatidylserine. EMBO J. 18, 6329–6338 (1999).
Dagda, R. K. et al. Loss of pink1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J. Biol. Chem. 284, 13843–13855 (2009).
Wild, P. et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 333, 228–233 (2011).
Kagan, V. E. et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 1, 223–232 (2005).
Tyurin, V. A. et al. Mass-spectrometric characterization of phospholipids and their primary peroxidation products in rat cortical neurons during staurosporine-induced apoptosis. J. Neurochem. 107, 1614–1633 (2008).
Zhu, J., Dagda, R. K. & Chu, C. T. Monitoring mitophagy in neuronal cell cultures. Methods Mol. Biol. 793, 325–339 (2011).
Yu, B. Z. et al. Structural basis of the anionic interface preference andkcat* activation of pancreatic phospholipase A2. Biochemistry 39, 12312–12323 (2000).
Nilsson, O. S. & Dallner, G. Transverse asymmetry of phospholipids in subcellular membranes of rat liver. Biochim. Biophys. Acta 464, 453–458 (1977).
Frezza, C., Cipolat, S. & Scorrano, L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc. 2, 287–295 (2007).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Sanner, M. F. Python: a programming language for software integration and development. J. Mol. Graph. Model. 17, 57–61 (1999).
Boeckmann, B. et al. The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res. 31, 365–370 (2003).
Thompson, J. D., Gibson, T. J. & Higgins, D. G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinformatics 2.3.1–2.3.22.
Crooks, G. E., Hon, G. & Chandonia, J. M. WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 (2004).
Acknowledgements
This study was supported in part by the National Institutes of Health (AG026389 (C.T.C.), NS065789 (C.T.C.), F32AG030821 (R.K.D.), NS061817 (H.B.), HL70755 (V.E.K.), OH008282 (V.E.K.), U19AIO68021 (H.B., V.E.K.), NS076511 (H.B., V.E.K.), ES020693 (V.E.K., Y.Y.T.)). We thank many generous scientists listed in the Methods for gifts of reagents, and D. E. Winnica for assistance with primary neuron cultures.
Author information
Authors and Affiliations
Contributions
J.J., R.K.D. and J.F.J. conducted and analysed experiments in the primary neuron, SH-SY5Y and HeLa cell systems, respectively. A.A.A., Y.Y.T. and V.A.T. collected and analysed the LC–MS data. K.Z.Q.W. contributed to LC3 mutagenesis and PLS3 knockdown studies. A.A.K performed the liposome studies. D.M., N.Y., J.K-S., I.H.S. and I.B. performed computational modelling. K.B., G.B. and Z.H. developed and optimized specific assays for the study. C.B. and S.W. performed specialized fluorescence microscopy. J.Z., A.M.G., E.K.S., R.W. and A.C. contributed to the PINK1, parkin, p62 and flux analyses. C.T.C., H.B. and V.E.K. planned and designed the study, analysed data, developed the conceptual model and wrote the manuscript. All authors discussed the results, and contributed to writing or commenting on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Additional characterization of Rot and STS-induced mitophagy.
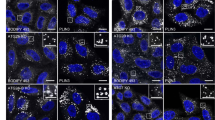
Primary rat cortical neuron cultures were treated with rotenone at 7–9 days in vitro (DIV) using the indicated doses and times. Cell death was analyzed by flow cytometry of Annexin V/propidium iodide stained neurons to define sub-lethal conditions (a). N = 6 independent experiments; mean +/− s.d., *p<0.05 vs. vehicle. Equivalent results were observed for caspase-3/7 activation (not shown), assayed using the luminescent Promega Caspase-Glo assay (Madison, WI). Rotenone elicited increased GFP-LC3 puncta (b) and colocalization with mitochondria (c), the latter of which was inhibited by siRNA knockdown of CLS (siCLS), but not scrambled siCtrl. For b,c: N = 3; mean +/− s.d. *p<0.05 vs. vehicle-treated neurons; †p<0.05 vs. respective Rot-treated siCtrl neurons. Primary cortical neurons treated with vehicle or the indicated concentrations of rotenone were analyzed for p62 protein levels by immunoblot. Densitometry indicates significant decreases in p62 levels, consistent with dose-dependent increases in autophagic flux (d). N = 3; mean +/− s.d. *p<0.01 vs. vehicle; **p<0.01 vs. Rot 125 nM. Densitometry of mitochondrial proteins analyzed by immunoblot (See Fig. 1g) confirmed decreased protein levels, inhibited by co-treatment with bafilomycin (e). N = 3; mean +/− s.d., *p<0.05 vs. Ctrl; †p<0.05 vs. Rot alone. Primary neurons transfected on DIV 4 with siCLS or siCtrl were treated with STS on DIV 7 and analyzed for autophagy by LC3 shift (f) and GFP-LC3 puncta (g), and for mitophagy by colocalization analysis (h) or by loss of mitochondrial proteins (f). For g,h: N = 3; mean +/− s.d. *p<0.05 vs. respective vehicle-treated neurons; †p<0.05 vs. STS-treated siCtrl neurons. STS also elicited mitophagy in SH-SY5Y cells (i), as assessed by delivery of mitochondria to LTR-labeled lysosomes, which was inhibited by siRNA knockdown of the autophagy proteins ATG7 or LC3, as well as PLS3. *p<0.05 vs Veh; †p<0.05 vs STS/siCtrl.
Supplementary Figure 2 Mitochondrial isolation and characterization of phospholipid content and accessibility.
Primary cortical neuron cultures (DIV 7 or 8) were exposed to rotenone followed by isolation of mitochondrial IMM and OMM for LC-MS analysis. Immunoblot analysis employed the following (a): COXIV - IMM marker; TOM40 - OMM marker; pan Cadherin - cellular membrane marker; Histone H3 - nuclear marker. Western blot analysis for contact site IMM and OMM proteins ANT and VDAC, respectively, reveal no significant cross-contamination of fractions (b, Rot, 250 nM×2 h, representative of 3 experiments). Neuronal cells were treated with rotenone (250 nM) or STS (100 nM), and the IMM and OMM fractions prepared from isolated mitochondria were pooled from 3 experiments for lipid extraction and LC-MS analysis as described in Methods. Representative spectra for phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), and phosphatidic acid (PA) are shown for mitochondrial membranes isolated from control, rotenone- and STS-treated neurons are shown (c). In contrast to CL (Fig. 2a), only subtle changes in peak distribution, intensity, or cluster pattern of IMM and OMM PC, PE, PI and PA were observed. Isolated liver mitochondria labeled with the indicated phospholipids were mixed with Annexin V in the presence of the indicated Ca2+ concentrations. Annexin V binding was assessed by flow cytometry, indicating specificity for CL at lower Ca2+ concentrations (d). The highest concentration shown is equivalent to 300 μM. N = 4 experiments, mean +/− s.d. STS-treated HeLa cells exhibited increased surface accessibility of CL on isolated mitochondria probed with Annexin V (e). Inset: purity of membrane fractions. *p<0.05vs. control. N = 3 experiments, mean +/− s.d.
Supplementary Figure 3 Additional characterization of phospholipid scramblase 3 (PLS3) knockdowns.
Mitochondrial and cytosolic fractions from SH-SY5Y cells were immunoblotted for human scramblase-3 (hPLS3), and for the human mitochondrial antigen of 60 kDa (mito-P60) or the cytosolic lactate dehydrogenase enzyme (LDH) as compartment markers (a). SH-SY5Y cells were treated with two siRNAs targeting base pairs 433-453 (#433) or base pairs 508-532 (#508), and the extent of knockdown assessed by immunoblot densitometry (b). n = 6, ‡p<0.05vs. siCtrl. RNAi knockdown of PLS3 (siPLS3#508) blocks the Rot-induced colocalization of mitochondria with LTR-labeled lysosomes (c). *p<0.05 vs. Veh/siCtrl; †p<0.05 vs. Rot/siCtrl. Western blot analysis of the effects of siPLS3 on Rot-induced decreases in COX IV and MnSOD (d). Effects of PLS3 knockdown on basal and 6-OHDA-elicited GFP-LC3 puncta (e). *p<0.05 vs. respective vehicle treated cells. There were no significant effects of PLS3 knockdown on baseline or rotenone-treated mitochondrial membrane potentials (f). n = 8 wells/condition, representative of two independent experiments, no significant changes by ANOVA. The two siRNAs showed equivalent effects on mitophagy elicited by Rot (g)or by 6-OHDA (Fig. 3d). *p<0.05 vs. Veh/siCtrl; †p<0.05 vs. Rot/siCtrl.
Supplementary Figure 4 Additional characterization of cardiolipin synthase (CLS) knockdowns.
Treatment of primary cortical neurons with siCLS resulted in knockdown of rat CLS without affecting expression of other mitochondrial proteins (a). N = 3, mean +/− s.d. *p<0.05 vs. siCtrl. CLS knockdown had no effects on membrane potential measured by TMRM staining, and did not affect the small, but significant decrease elicited by rotenone (b). N = 3, *p<0.05 vs. Veh. CLS knockdown suppressed the loss of mitochondrial proteins elicited by rotenone as quantified by densitometry (c). N = 3; *p<0.05 vs. vehicle; †p<0.05 vs. respective Rot/siCtrl. See also Fig. 3g–i for representative blots and additional quantification. A second independent CLS siRNA (siCLS#2) was also effective in reducing CLS expression (d) and inhibiting the loss of mitochondrial proteins (e), with no effect on baseline or rotenone-treated membrane potentials (f). N = 3, *p<0.05 vs. Veh.
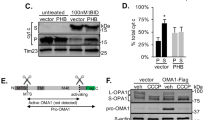
Supplementary Figure 5 The potential relationship of CL to the FCCP/CCCP-PINK1-Parkin pathway.
A previously characterized stable PINK1-3xFlag expressing SH-SY5Y line (#24, Reference 10), was treated with FCCP (2 μM×4 hrs) beginning at 72 h after transfection with siCtrl or siPLS3. Immunoblot analysis confirms PLS3 knockdown, which had no effect on the well-characterized FCCP-induced accumulation of full-length PINK1 (a). SH-SY5Y cells were co-transfected with HA-Parkin and siCtrl or siPLS3, treated with FCCP 72 h later, fixed in paraformaldehyde and immunolabeled for HA (green) and mitochondrial p60 antigen (red). Scale bar: 10 μm. FCCP elicited HA-Parkin puncta, all of which colocalized with mitochondrial p60 (b, arrows). There were no effects of siPLS3 on basal or FCCP-induced Parkin puncta (c), nor were there effects on FCCP-induced mitochondrial translocation of Parkin analyzed by immunoblot (not shown). *p ¡0.05 vs. respective Veh-treated cells. While PLS3 knockdown had no effect on FCCP-induced autophagosomes (d), it significantly reduced the degree of mitophagy elicited by FCCP (e). Overexpression of Drp1 did not rescue the inhibition of mitophagy by PLS knockdown (e), despite promoting significant further mitochondrial fragmentation (not shown). * p¡0.05 vs. respective Veh-treated cells; †p<0.05 vs. FCCP/siCtrl. Similarly, in Parkin-expressing HeLa cells, knockdown of CLS (siCLS #S29308) did not block the mitochondrial translocation of Parkin (f). Nevertheless, CCCP treatment did elicit higher levels of CL at the mitochondrial surface, as assessed using both the AnnexinV assay (g) and the PLA2 hydrolysis assay (h), which was inhibited by siCLS. *p<0.05 vs. Veh/siCtrl. †p<0.05 vs. CCCP/siCLS. CCCP treatment of Parkin-expressing HeLa cells resulted in the loss of mitochondrial proteins MnSOD and TOM40, while RNAi knockdown of CLS partially reduced this loss (i). Representative of 3 independent experiments. Effective knockdown of human CLS was demonstrated in Parkin-transfected HeLa cells at 72 h after siRNA treatment (j), which was associated with a ∼ 30% decrease in the levels of total CL in these cells (k).
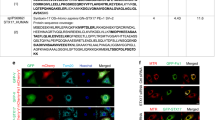
Supplementary Figure 6 Dynamic modeling of LC3/CL interactions.
LC3 and three closest CL molecules in simulation MD1 are shown at four successive snapshots (a). Water, ions and POPC molecules are not shown in the snapshots for clarity. Note that LC3 enters the interaction range (less than 6 Å) of CL within 10 ns in all three simulations. Time evolution of LC3 position (b), monitored as minimum distances between head group atoms on CL and residues R68-R70 (black), R10-R11 (red), K42 (green) or K5 (blue) as observed in each of the three simulations (MD1-3). Magnified snapshots illustrate LC3/CL binding poses with the indicated residues at the end of 50 ns in each of the three simulations (c). For clarity, only those CL molecules which interact with LC3 are illustrated. The LC3 residues interacting with CL are highlighted in stick representation and labeled. The number of CL, PA and LysoCL interacting with LC3 is graphed with time during CGMD simulations (d). Insert snapshots illustrate the simulated interaction of LC3 with a PA-containing bilayer. Color guide: DOPC: yellow; head groups of PA: dark blue; acyl chain of PA: light blue; LC3: purple. CGMD simulation of the interaction of CL with LC3 for 1 μs (e). Snapshots illustrate the simulated interaction of LC3 with a CL-containing membrane using elastic network to preserve the protein structure (Refs. 9-10, SI Note). Note that water, ions and DOPC molecules are not shown for clarity. Color guide: head groups of CL: dark blue sticks, acyl chain of CL: light blue sticks, LC3: cyan (transparent), N-terminus of LC3 (K5, R10-R11): blue spheres, K49, R68-R69-R70: green spheres, Glu117: red sphere). Arrow indicates the C-terminal region involved in lipidation.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2508 kb)
Supplementary Table 1
Supplementary Information (XLSX 27 kb)
Rights and permissions
About this article
Cite this article
Chu, C., Ji, J., Dagda, R. et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol 15, 1197–1205 (2013). https://doi.org/10.1038/ncb2837
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2837
This article is cited by
-
Therapeutic Strategies Targeting Mitochondrial Dysfunction in Sepsis-induced Cardiomyopathy
Cardiovascular Drugs and Therapy (2024)
-
Mitophagy plays a “double-edged sword” role in the radiosensitivity of cancer cells
Journal of Cancer Research and Clinical Oncology (2024)
-
Recent advances on the molecular mechanisms of exercise-induced improvements of cognitive dysfunction
Translational Neurodegeneration (2023)
-
Mitochondrial dysfunction: roles in skeletal muscle atrophy
Journal of Translational Medicine (2023)
-
Mitophagy associated self-degradation of phosphorylated MAP4 guarantees the migration and proliferation responses of keratinocytes to hypoxia
Cell Death Discovery (2023)