Abstract
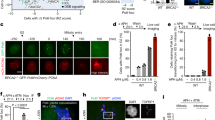
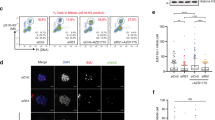
The essential checkpoint kinase Chk1 is required for cell-cycle delays after DNA damage or blocked DNA replication1,2. However, it is unclear whether Chk1 is involved in the repair of damaged DNA. Here we establish that Chk1 is a key regulator of genome maintenance by the homologous recombination repair (HRR) system. Abrogation of Chk1 function with small interfering RNA or chemical antagonists inhibits HRR, leading to persistent unrepaired DNA double-strand breaks (DSBs) and cell death after replication inhibition with hydroxyurea or DNA-damage caused by camptothecin. After hydroxyurea treatment, the essential recombination repair protein RAD51 is recruited to DNA repair foci performing a vital role in correct HRR3,4. We demonstrate that Chk1 interacts with RAD51, and that RAD51 is phosphorylated on Thr 309 in a Chk1-dependent manner. Consistent with a functional interplay between Chk1 and RAD51, Chk1-depleted cells failed to form RAD51 nuclear foci after exposure to hydroxyurea, and cells expressing a phosphorylation-deficient mutant RAD51T309A were hypersensitive to hydroxyurea. These results highlight a crucial role for the Chk1 signalling pathway in protecting cells against lethal DNA lesions through regulation of HRR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Abraham, R. T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15, 2177–2196 (2001).
Bartek, J. & Lukas, J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3, 421–429 (2003).
West, S. C. Molecular views of recombination proteins and their control. Nature Rev. Mol. Cell Biol. 4, 435–445 (2003).
Saintigny, Y. et al. Characterization of homologous recombination induced by replication inhibition in mammalian cells. EMBO J. 20, 3861–3870 (2001).
Liu, Q. et al. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 14, 1448–1459 (2000).
Takai, H. et al. Aberrant cell cycle checkpoint function and early embryonic death in Chk1−/− mice. Genes Dev. 14, 1439–1447 (2000).
Zhao, H. & Piwnica-Worms, H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol. 21, 4129–4139 (2001).
Brown, E. J. & Baltimore, D. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 17, 615–628 (2003).
Casper, A. M., Nghiem, P., Arlt, M. F. & Glover, T. W. ATR regulates fragile site stability. Cell 111, 779–789 (2002).
Katou, Y. et al. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature 424, 1078–1083 (2003).
Tercero, J. A. & Diffley, J. F. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature 412, 553–557 (2001).
Lopes, M. et al. The DNA replication checkpoint response stabilizes stalled replication forks. Nature 412, 557–561 (2001).
Feijoo, C. et al. Activation of mammalian Chk1 during DNA replication arrest: a role for Chk1 in the intra-S phase checkpoint monitoring replication origin firing. J. Cell Biol. 154, 913–923 (2001).
Johnson, R. D. & Jasin, M. Sister chromatid gene conversion is a prominent double-strand break repair pathway in mammalian cells. EMBO J. 19, 3398–3407 (2000).
Lundin, C. et al. Different roles for nonhomologous end joining and homologous recombination following replication arrest in mammalian cells. Mol. Cell. Biol. 22, 5869–5878 (2002).
Sonoda, E. et al. Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J. 17, 598–608 (1998).
Arnaudeau, C., Lundin, C. & Helleday, T. DNA double-strand breaks associated with replication forks are predominantly repaired by homologous recombination involving an exchange mechanism in mammalian cells. J. Mol. Biol. 307, 1235–1245 (2001).
Cliby, W. A. et al. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J. 17, 159–169 (1998).
Sarkaria, J. N. et al. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59, 4375–4382 (1999).
Busby, E. C., Leistritz, D. F., Abraham, R. T., Karnitz, L. M. & Sarkaria, J. N. The radiosensitizing agent 7-hydroxystaurosporine (UCN-01) inhibits the DNA damage checkpoint kinase hChk1. Cancer Res. 60, 2108–2112 (2000).
Mohindra, A. et al. Defects in homologous recombination repair in mismatch-repair-deficient tumour cell lines. Hum. Mol. Genet. 11, 2189–2200 (2002).
Tebbs, R. S. et al. Correction of chromosomal instability and sensitivity to diverse mutagens by a cloned cDNA of the XRCC3 DNA repair gene Proc. Natl Acad. Sci. USA 92, 6354–6358 (1995).
Jackson, S. P. Sensing and repairing DNA double-strand breaks. Carcinogenesis 23, 687–696 (2002).
Sørensen, C. S. et al. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell 3, 247–258 (2003).
Saleh-Gohari, N. & Helleday, T. Conservative homologous recombination preferrentially repairs DNA double-strand breaks in the S phase of the cell cycle in human cells. Nucleic Acid Res. 32, 3683–3688 (2004).
Haaf, T., Golub, E. I., Reddy, G., Radding, C. M. & Ward, D. C. Nuclear foci of mammalian Rad51 recombination protein in somatic cells after DNA damage and its localization in synaptonemal complexes. Proc. Natl Acad. Sci. USA 92, 2298–2302 (1995).
Sigurdsson, S. et al. Mediator function of the human Rad51B–Rad51C complex in Rad51/RPA-catalyzed DNA strand exchange. Genes Dev. 15, 3308–3318 (2001).
Brown, E. J. & Baltimore, D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 14, 397–402 (2000).
de Klein, A. et al. Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr. Biol. 10, 479–482 (2000).
Tsuzuki, T. et al. Targeted disruption of the Rad51 gene leads to lethality in embryonic mice. Proc. Natl Acad. Sci. USA 93, 6236–6240 (1996).
Luo, G. et al. Disruption of mRad50 causes embryonic stem cell lethality, abnormal embryonic development, and sensitivity to ionizing radiation. Proc. Natl Acad. Sci. USA 96, 7376–7381 (1999).
Deans, B., Griffin, C. S., Maconochie, M. & Thacker, J. Xrcc2 is required for genetic stability, embryonic neurogenesis and viability in mice. EMBO J. 19, 6675–6685 (2000).
Acknowledgements
We thank M. Jasin for the SCneo and pCMV3xnlsI-SceI vectors, L. Thompson for cell lines and the Danish Cancer Society, the Novo Nordisk Foundation, the European Union, the Danish Medical Research Council, Dansk Kraeftforskningsfond, the Swedish Cancer Society, the Swedish Research Council, the Biological & Biotechnological Sciences Research Council and Yorkshire Cancer Research for grant support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Fig S1, Fig S2, Fig S3, Table S1 (PDF 427 kb)
Rights and permissions
About this article
Cite this article
Sørensen, C., Hansen, L., Dziegielewski, J. et al. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol 7, 195–201 (2005). https://doi.org/10.1038/ncb1212
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1212
This article is cited by
-
Integrative modeling uncovers p21-driven drug resistance and prioritizes therapies for PIK3CA-mutant breast cancer
npj Precision Oncology (2024)
-
BRD7 suppresses tumor chemosensitivity to CHK1 inhibitors by inhibiting USP1-mediated deubiquitination of CHK1
Cell Death Discovery (2023)
-
Targeting replication stress in cancer therapy
Nature Reviews Drug Discovery (2023)
-
CENPF knockdown inhibits adriamycin chemoresistance in triple-negative breast cancer via the Rb-E2F1 axis
Scientific Reports (2023)
-
In Silico design and characterization of RAD51 protein inhibitors targeting homologous recombination repair for cancer therapy
Genome Instability & Disease (2023)