Abstract
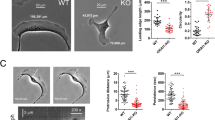
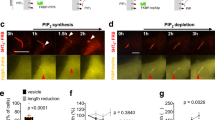
Polarized cell movement is triggered by the development of a PtdIns(3,4,5)P3 gradient at the membrane, which is followed by rearrangement of the actin cytoskeleton. The WASP family verprolin homologous protein (WAVE) is essential for lamellipodium formation at the leading edge by activating the Arp2/3 complex downstream of Rac GTPase. Here, we report that WAVE2 binds to PtdIns(3,4,5)P3 through its basic domain. The amino-terminal portion of WAVE2, which includes the PtdIns(3,4,5)P3-binding sequence, was localized at the leading edge of lamellipodia induced by an active form of Rac (RacDA) or by treatment with platelet-derived growth factor (PDGF). Production of PtdIns(3,4,5)P3 at the cell membrane by myristoylated phosphatidylinositol-3-OH kinase (PI(3)K) is sufficient to recruit WAVE2 in the presence of dominant-negative Rac and latrunculin, demonstrating that PtdIns(3,4,5)P3 alone is able to recruit WAVE2. Expression of a full-length mutant of WAVE2 that lacks the lipid-binding activity inhibited proper formation of lamellipodia induced by RacDA. These results suggest that one of the products of PI(3)K, PtdIns(3,4,5)P3, recruits WAVE2 to the polarized membrane and that this recruitment is essential for lamellipodium formation at the leading edge.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rickert, P., Weiner, O.D., Wang, F., Bourne, H.R. & Servant, G. Leukocytes navigate by compass: roles of PI(3)Kgamma and its lipid products. Trends Cell Biol. 10, 466–473 (2000).
Insall, R.H. & Weiner, O.D. PIP3, PIP2, and cell movement — similar messages, different meanings? Dev. Cell 1, 743–747 (2001).
Chung, C.Y., Funamoto, S. & Firtel, R.A. Signaling pathways controlling cell polarity and chemotaxis. Trends Biochem. Sci. 26, 557–566 (2001).
Pollard, T.D. & Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 (2003).
Nobes, C.D. & Hall, A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 144, 1235–1244 (1999).
Ridley, A.J. Rho GTPases and cell migration. J. Cell Sci. 114, 2713–2722 (2001).
Hall, A. Rho GTPases and the actin cytoskeleton. Science 279, 509–514 (1998).
Takenawa, T. & Miki, H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J. Cell Sci. 114, 1801–1809 (2001).
Prehoda, K.E., Scott, J.A., Mullins, R.D. & Lim, W.A. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science 290, 801–806 (2000).
Rohatgi, R., Ho, H.Y. & Kirschner, M.W. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J. Cell Biol. 150, 1299–1310 (2000).
Miki, H., Suetsugu, S. & Takenawa, T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 17, 6932–6941 (1998).
Miki, H., Yamaguchi, H., Suetsugu, S. & Takenawa, T. IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature 408, 732–735 (2000).
Suetsugu, S., Miki, H. & Takenawa, T. Identification of two human WAVE/SCAR homologues as general actin regulatory molecules which associate with the Arp2/3 complex. Biochem. Biophys. Res. Commun. 260, 296–302 (1999).
Yamazaki, D. et al. WAVE2 is required for directed cell migration and cardiovascular development. Nature 424, 452–456 (2003).
Yan, C. et al. WAVE2 deficiency reveals distinct roles in embryogenesis and Rac-mediated actin-based motility. EMBO J. 22, 3602–3612 (2003).
Suetsugu, S., Yamazaki, D., Kurisu, S. & Takenawa, T. Differential roles of WAVE1 and WAVE2 in dorsal and peripheral ruffle formation for fibroblast cell migration. Dev. Cell 5, 595–609 (2003).
Takenawa, T. & Itoh, T. Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim. Biophys. Acta 1533, 190–206 (2001).
Cross, G.H. et al. A new quantitative optical biosensor for protein characterisation. Biosens. Bioelectron. 19, 383–390 (2003).
Benard, V., Bohl, B.P. & Bokoch, G.M. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274, 13198–13204 (1999).
Weiner, O.D. Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr. Opin. Cell Biol. 14, 196–202 (2002).
Wang, F. et al. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nature Cell Biol. 4, 513–518 (2002).
Weiner, O.D. et al. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nature Cell Biol. 4, 509–513 (2002).
Kitamura, T. et al. Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Mol. Cell Biol. 19, 6286–6296 (1999).
Tengholm, A. & Meyer, T. A PI3-kinase signaling code for insulin-triggered insertion of glucose transporters into the plasma membrane. Curr. Biol. 12, 1871–1876 (2002).
Srinivasan, S. et al. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J. Cell Biol. 160, 375–385 (2003).
Servant, G. et al. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287, 1037–1040 (2000).
Iijima, M. & Devreotes, P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell 109, 599–610 (2002).
Funamoto, S., Meili, R., Lee, S., Parry, L. & Firtel, R.A. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell 109, 611–623 (2002).
Eden, S., Rohatgi, R., Podtelejnikov, A.V., Mann, M. & Kirschner, M.W. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature 418, 790–793 (2002).
Steffen, A. et al. Sra-1 and Nap1 link Rac to actin assembly driving lamellipodia formation. EMBO J. 23, 749–759 (2004).
Acknowledgements
We thank M. Kasuga for providing myristoylated PI(3)K-p110α, and M. Furutani and K. Tsujita for discussions and the PLCδ1PH constructs. This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, and Technology of Japan, and from the Japan Science and Technology Corporation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary figures
Supplementary Information, Fig. S1 (PDF 808 kb)
Supplementary Information, Table S1
Rights and permissions
About this article
Cite this article
Oikawa, T., Yamaguchi, H., Itoh, T. et al. PtdIns(3,4,5)P3 binding is necessary for WAVE2-induced formation of lamellipodia. Nat Cell Biol 6, 420–426 (2004). https://doi.org/10.1038/ncb1125
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1125
This article is cited by
-
Orchestration of synaptic functions by WAVE regulatory complex-mediated actin reorganization
Experimental & Molecular Medicine (2023)
-
Regulation of the Scar/WAVE complex in migrating cells: A summary of our understanding
Journal of Biosciences (2023)
-
Increased spine PIP3 is sequestered from dendritic shafts
Molecular Brain (2022)
-
Endosomal PI(3)P regulation by the COMMD/CCDC22/CCDC93 (CCC) complex controls membrane protein recycling
Nature Communications (2019)
-
Suppression of cell migration by phospholipase C-related catalytically inactive protein-dependent modulation of PI3K signalling
Scientific Reports (2017)