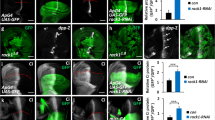
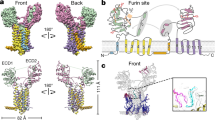
Abstract
The mechanisms involved in transduction of the Hedgehog (Hh) signal are of considerable interest to developmental and cancer biologists. Stabilization of the integral membrane protein Smoothened (Smo) at the plasma membrane is a crucial step in Hh signalling but the molecular events immediately downstream of Smo remain to be elucidated. We have shown previously that the transcriptional mediator Cubitus interruptus (Ci) is associated in a protein complex with at least two other proteins, the kinesin-like Costal2 (Cos2) and the serine–threonine kinase Fused (Fu). This protein complex governs the access of Ci to the nucleus. Here we show that, consequent on the stabilization of Smo, Cos2 and Fu are destabilized. Moreover, we find that the Cos2–Fu–Ci protein complex is associated with Smo in membrane fractions both in vitro and in vivo. We also show that Cos2 binding on Smo is necessary for the Hh-dependent dissociation of Ci from this complex. We propose that the association of the Cos2 protein complex with Smo at the plasma membrane controls the stability of the complex and allows Ci activation, eliciting its nuclear translocation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ingham, P.W. & McMahon, A.P. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15, 3059–3087 (2001).
Tabata, T., Eaton, S. & Kornberg, T.B. The Drosophila hedgehog gene is expressed specifically in posterior compartment cells and is a target of engrailed regulation. Genes Dev. 6, 2635–2645 (1992).
Lee, J.J., von Kessler, D.P., Parks, S. & Beachy, P.A. Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell 71, 33–50 (1992).
Dominguez, M., Brunner, M., Hafen, E. & Basler, K. Sending and receiving the hedgehog signal: control by the Drosophila Gli protein Cubitus interruptus. Science 272, 1621–1625 (1996).
Ingham, P.W., Taylor, A.M. & Nakano, Y. Role of the Drosophila patched gene in positional signalling. Nature 353, 184–187 (1991).
Marigo, V., Davey, R.A., Zuo, Y., Cunningham, J.M. & Tabin, C.J. Biochemical evidence that patched is the Hedgehog receptor. Nature 384, 176–179 (1996).
Alcedo, J., Ayzenzon, M., Von Ohlen, T., Noll, M. & Hooper, J.E. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell 86, 221–232 (1996).
van den Heuvel, M. & Ingham, P.W. smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nature 382, 547–551 (1996).
Chen, Y. & Struhl, G. Dual roles for patched in sequestering and transducing Hedgehog. Cell 87, 553–563 (1996).
Denef, N., Neubuser, D., Perez, L. & Cohen, S.M. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell 102, 521–531 (2000).
Zhu, A.J. et al. Altered localization of Drosophila Smoothened protein activates Hedgehog signal transduction. Genes Dev. 17, 1240–1252 (2003).
Robbins, D.J. et al. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell 90, 225–234 (1997).
Sisson, J.C., Ho, K.S., Suyama, K. & Scott, M.P. Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell 90, 235–245 (1997).
Wang, G., Amanai, K., Wang, B. & Jiang, J. Interactions with Costal2 and Suppressor of fused regulate nuclear translocation and activity of Cubitus interruptus. Genes Dev. 14, 2893–2905 (2000).
Alcedo, J., Zou, Y. & Noll, M. Posttranscriptional regulation of smoothened is part of a self-correcting mechanism in the Hedgehog signaling system. Mol. Cell 6, 457–465 (2000).
Ingham, P.W. et al. Patched represses the Hedgehog signalling pathway by promoting modification of the Smoothened protein. Curr. Biol. 10, 1315–1318 (2000).
Therond, P. et al. Molecular organisation and expression pattern of the segment polarity gene fused of Drosophila melanogaster. Mech. Dev. 44, 65–80 (1993).
Martin, V., Carrillo, G., Torroja, C. & Guerrero, I. The sterol-sensing domain of Patched protein seems to control Smoothened activity through Patched vesicular trafficking. Curr. Biol. 11, 601–607 (2001).
Strigini, M. & Cohen, S.M. A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development 124, 4697–4705 (1997).
Hooper, J.E. Smoothened translates Hedgehog levels into distinct responses. Development 130, 3951–3963 (2003).
Therond, P.P., Knight, J.D., Kornberg, T.B. & Bishop, J.M. Phosphorylation of the fused protein kinase in response to signaling from hedgehog. Proc. Natl Acad. Sci. USA 93, 4224–4228 (1996).
Chen, C.H. et al. Nuclear trafficking of Cubitus interruptus in the transcriptional regulation of Hedgehog target gene expression. Cell 98, 305–316 (1999).
Sasaki, H., Hui, C., Nakafuku, M. & Kondoh, H. A binding site for Gli proteins is essential for HNF-3β floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development 124, 1313–1322 (1997).
Clemens, J.C. et al. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl Acad. Sci. USA 97, 6499–6503 (2000).
Morfini, G., Szebenyi, G., Elluru, R., Ratner, N. & Brady, S.T. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 21, 281–293 (2002).
Muller, H.A. & Wieschaus, E. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J. Cell Biol. 134, 149–163 (1996).
Monnier, V., Ho, K.S., Sanial, M., Scott, M.P. & Plessis, A. Hedgehog signal transduction proteins: contacts of the Fused kinase and Ci transcription factor with the kinesin-related protein Costal2. BMC Dev. Biol. 2, 4–13 (2002).
Price, M.A., Kalderon, D. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by glycogen synthase kinase 3 and casein kinase 1. Cell 108, 823–835 (2002).
Basler, K. & Struhl, G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature 368, 208–214 (1994).
Gallet, A., Rodriguez, R., Ruel, L. & Therond, P.P. Cholesterol modification of hedgehog is required for trafficking and movement, revealing an asymmetric cellular response to hedgehog. Dev. Cell 4, 191–204 (2003).
Strutt, H. et al. Mutations in the sterol-sensing domain of Patched suggest a role for vesicular trafficking in Smoothened regulation. Curr. Biol. 11, 608–613 (2001).
van Leeuwen, F., Samos, C.H. & Nusse, R. Biological activity of soluble wingless protein in cultured Drosophila imaginal disc cells. Nature 368, 342–344 (1994).
Acknowledgements
We thank A. Le Bivic and S. Cohen for antibodies; M. van den Heuvel, K. Ho, M. Scott, K. Basler, P. Ingham, M. Gonzalez-Gaitan and I. Guerrero for fly stocks; all 'fly' members of the ISDBCR for exciting discussions about this work; and P. Léopold, J. P. Vincent, S. Eaton, A. Goldsborough and N. Tapon for comments on the manuscript. R.R. is supported by a doctoral fellowship from the French Research Ministry. This work was supported by grants from the 'Association pour la Recherche sur le Cancer', 'la Fondation de France' and the ATIPE programme of the Centre National de la Recherche Scientifique to P.P.T.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Ruel, L., Rodriguez, R., Gallet, A. et al. Stability and association of Smoothened, Costal2 and Fused with Cubitus interruptus are regulated by Hedgehog. Nat Cell Biol 5, 907–913 (2003). https://doi.org/10.1038/ncb1052
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1052
This article is cited by
-
Control of the Hedgehog pathway by compartmentalized PKA in the primary cilium
Science China Life Sciences (2022)
-
The Drosophila TNF receptor Grindelwald couples loss of cell polarity and neoplastic growth
Nature (2015)
-
Smoothened regulation in response to Hedgehog stimulation
Frontiers in Biology (2015)
-
SPOP suppresses tumorigenesis by regulating Hedgehog/Gli2 signaling pathway in gastric cancer
Journal of Experimental & Clinical Cancer Research (2014)
-
Switch of PKA substrates from Cubitus interruptus to Smoothened in the Hedgehog signalosome complex
Nature Communications (2014)