Abstract
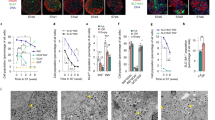
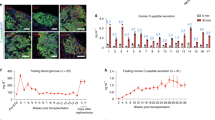
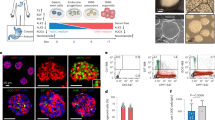
Transplantation of pancreatic progenitors or insulin-secreting cells derived from human embryonic stem cells (hESCs) has been proposed as a therapy for diabetes. We describe a seven-stage protocol that efficiently converts hESCs into insulin-producing cells. Stage (S) 7 cells expressed key markers of mature pancreatic beta cells, including MAFA, and displayed glucose-stimulated insulin secretion similar to that of human islets during static incubations in vitro. Additional characterization using single-cell imaging and dynamic glucose stimulation assays revealed similarities but also notable differences between S7 insulin-secreting cells and primary human beta cells. Nevertheless, S7 cells rapidly reversed diabetes in mice within 40 days, roughly four times faster than pancreatic progenitors. Therefore, although S7 cells are not fully equivalent to mature beta cells, their capacity for glucose-responsive insulin secretion and rapid reversal of diabetes in vivo makes them a promising alternative to pancreatic progenitor cells or cadaveric islets for the treatment of diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
16 September 2014
Erratum: In the version of this article initially published online, there were several errors in the PDF and/or the HTML versions. PDF and HTML versions: In the abstract, the last sentence should have read “in vivo” rather than “in vitro.” In the Methods, under the heading “Differentiation of S4 cells in suspension aggregate cultures,” the units of the cell clump size should have been micrometers rather than millimeters, as follows: “On the last day of culture, S4 cells were treated with 5 mg/ml dispase (Corning, Cat# 354235) for 5 min at 37 °C, followed by gentle pipetting to break into cell clumps (100–200 μm).” In the Methods, under the heading “S4: pancreatic endoderm, PDX1+/NKX6.1+ cells (3 d),” milliliter should have been microliter, as follows: “The resulting cell pellet was resuspended as single cells at a density of ~0.5 × 105 cells/μl on filter inserts (BD, Cat#35-3493 or Corning Cat#3420); 5–10 μl per spot for a total of 0.25–0.5 × 106 cells/spot) at an air-liquid interface.” HTML version: In the legends to Figures 2, 3, 4 and 7, the units of the scale bars should have been micrometers rather than millimeters. The errors have been corrected for the print, PDF and HTML versions of this article.
References
Barton, F.B. et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 35, 1436–1445 (2012).
Warnock, G.L. et al. A multi-year analysis of islet transplantation compared with intensive medical therapy on progression of complications in type 1 diabetes. Transplantation 86, 1762–1766 (2008).
Bruin, J.E. et al. Characterization of polyhormonal insulin-producing cells derived in vitro from human embryonic stem cells. Stem Cell Res. 12, 194–208 (2014).
Bruin, J.E. et al. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia 56, 1987–1998 (2013).
Rezania, A. et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 61, 2016–2029 (2012).
Rezania, A. et al. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells 31, 2432–2442 (2013).
Rezania, A. et al. Production of functional glucagon-secreting alpha-cells from human embryonic stem cells. Diabetes 60, 239–247 (2011).
Chen, S. et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat. Chem. Biol. 5, 258–265 (2009).
Xu, X., Browning, V.L. & Odorico, J.S. Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mech. Dev. 128, 412–427 (2011).
Shim, J.H. et al. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia 50, 1228–1238 (2007).
Cho, C.H. et al. Inhibition of activin/nodal signalling is necessary for pancreatic differentiation of human pluripotent stem cells. Diabetologia 55, 3284–3295 (2012).
D'Amour, K.A. et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24, 1392–1401 (2006).
Kroon, E. et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 26, 443–452 (2008).
Nostro, M.C. et al. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development 138, 861–871 (2011).
Kelly, O.G. et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat. Biotechnol. 29, 750–756 (2011).
Schiesser, J.V. & Wells, J.M. Generation of beta cells from human pluripotent stem cells: are we there yet? Ann. NY Acad. Sci. 1311, 124–137 (2014).
Nostro, M.C. & Keller, G. Generation of beta cells from human pluripotent stem cells: Potential for regenerative medicine. Semin. Cell Dev. Biol. 23, 701–710 (2012).
Halban, P.A., Kahn, S.E., Lernmark, A. & Rhodes, C.J. Gene and cell-replacement therapy in the treatment of type 1 diabetes: how high must the standards be set? Diabetes 50, 2181–2191 (2001).
Aguayo-Mazzucato, C. & Bonner-Weir, S. Stem cell therapy for type 1 diabetes mellitus. Nat. Rev. Endocrinol. 6, 139–148 (2010).
Wells, J.M. & Melton, D.A. Vertebrate endoderm development. Annu. Rev. Cell Dev. Biol. 15, 393–410 (1999).
Nelson, S.B., Schaffer, A.E. & Sander, M. The transcription factors Nkx6.1 and Nkx6.2 possess equivalent activities in promoting beta-cell fate specification in Pdx1+ pancreatic progenitor cells. Development 134, 2491–2500 (2007).
Sander, M. et al. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development 127, 5533–5540 (2000).
Rukstalis, J.M. & Habener, J.F. Neurogenin3: a master regulator of pancreatic islet differentiation and regeneration. Islets 1, 177–184 (2009).
Matsuoka, T.A. et al. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc. Natl. Acad. Sci. USA 101, 2930–2933 (2004).
Wang, H., Brun, T., Kataoka, K., Sharma, A.J. & Wollheim, C.B. MAFA controls genes implicated in insulin biosynthesis and secretion. Diabetologia 50, 348–358 (2007).
Zhang, C. et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol. Cell. Biol. 25, 4969–4976 (2005).
Hang, Y. et al. The MafA transcription factor becomes essential to islet beta-cells soon after birth. Diabetes 63, 1994–2005 (2014).
Choi, K.M. et al. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J. Biosci. Bioeng. 105, 586–594 (2008).
Johansson, K.A. et al. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev. Cell 12, 457–465 (2007).
Schulz, T.C. et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS ONE 7, e37004 (2012).
Pruniéras, M., Regnier, M. & Woodley, D. Methods for cultivation of keratinocytes with an air-liquid interface. J. Invest. Dermatol. 81, 28s–33s (1983).
Pezzulo, A.A. et al. The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L25–L31 (2011).
Heinis, M. et al. Oxygen tension regulates pancreatic beta-cell differentiation through hypoxia-inducible factor 1alpha. Diabetes 59, 662–669 (2010).
Cortijo, C., Gouzi, M., Tissir, F. & Grapin-Botton, A. Planar cell polarity controls pancreatic beta cell differentiation and glucose homeostasis. Cell Reports 2, 1593–1606 (2012).
Shi, Y. & Massagué, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113, 685–700 (2003).
Aguayo-Mazzucato, C. et al. Thyroid hormone promotes postnatal rat pancreatic beta-cell development and glucose-responsive insulin secretion through MAFA. Diabetes 62, 1569–1580 (2013).
Seymour, P.A. et al. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc. Natl. Acad. Sci. USA 104, 1865–1870 (2007).
Murtaugh, L.C., Stanger, B.Z., Kwan, K.M. & Melton, D.A. Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. USA 100, 14920–14925 (2003).
Apelqvist, A. et al. Notch signalling controls pancreatic cell differentiation. Nature 400, 877–881 (1999).
Pisania, A. et al. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab. Invest. 90, 1661–1675 (2010).
Street, C.N. et al. Islet graft assessment in the Edmonton Protocol: implications for predicting long-term clinical outcome. Diabetes 53, 3107–3114 (2004).
Piper, K. et al. Beta cell differentiation during early human pancreas development. J. Endocrinol. 181, 11–23 (2004).
Aguayo-Mazzucato, C. et al. Mafa expression enhances glucose-responsive insulin secretion in neonatal rat beta cells. Diabetologia 54, 583–593 (2011).
Haase, T.N. et al. Growth arrest specific protein (GAS) 6: a role in the regulation of proliferation and functional capacity of the perinatal rat beta cell. Diabetologia 56, 763–773 (2013).
Harmon, J.S. et al. beta-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology 150, 4855–4862 (2009).
Harmon, J.S., Stein, R. & Robertson, R.P. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J. Biol. Chem. 280, 11107–11113 (2005).
Mahadevan, J. et al. Ebselen treatment prevents islet apoptosis, maintains intranuclear Pdx-1 and MafA levels, and preserves beta-cell mass and function in ZDF rats. Diabetes 62, 3582–3588 (2013).
Merani, S., Toso, C., Emamaullee, J. & Shapiro, A.M. Optimal implantation site for pancreatic islet transplantation. Br. J. Surg. 95, 1449–1461 (2008).
Kumar, M. & Melton, D. Pancreas specification: a budding question. Curr. Opin. Genet. Dev. 13, 401–407 (2003).
Jørgensen, M.C. et al. An illustrated review of early pancreas development in the mouse. Endocr. Rev. 28, 685–705 (2007).
Kinkel, M.D. & Prince, V.E. On the diabetic menu: zebrafish as a model for pancreas development and function. BioEssays 31, 139–152 (2009).
D'Amour, K.A. et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23, 1534–1541 (2005).
Basford, C.L. et al. The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic beta cells. Diabetologia 55, 358–371 (2012).
Luciani, D.S. & Johnson, J.D. Acute effects of insulin on beta-cells from transplantable human islets. Mol. Cell. Endocrinol. 241, 88–98 (2005).
Misler, S., Barnett, D.W., Gillis, K.D. & Pressel, D.M. Electrophysiology of stimulus-secretion coupling in human beta-cells. Diabetes 41, 1221–1228 (1992).
Nauck, M.A. et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J. Clin. Endocrinol. Metab. 63, 492–498 (1986).
Davalli, A.M. et al. A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation 59, 817–820 (1995).
Tuch, B.E. Reversal of diabetes by human fetal pancreas. Optimization of requirements in the hyperglycemic nude mouse. Transplantation 51, 557–562 (1991).
Tuch, B.E. & Monk, R.S. Regulation of blood glucose to human levels by human fetal pancreatic xenografts. Transplantation 51, 1156–1160 (1991).
Johnson, J.D. et al. Different effects of FK506, rapamycin, and mycophenolate mofetil on glucose-stimulated insulin release and apoptosis in human islets. Cell Transplant. 18, 833–845 (2009).
Acknowledgements
This work was supported in part by funding from the JDRF, the Canadian Institutes of Health Research (CIHR) Regenerative Medicine and Nanomedicine Initiative, and the Stem Cell Network (SCN). J.E.B. was funded by a JDRF postdoctoral fellowship and L'Oreal Canada for Women in Science Research Excellence Fellowship. Y.H.C.Y. was the recipient of an NSERC Post Graduate Scholarship and M.M. was the recipient of a Mitacs Elevate Postdoctoral Fellowship. The CIHR Transplantation Training Program provided funding for J.E.B., N.Q. and M.M. We also thank Stem Cell Technologies for their financial support, T. Webber, S. Karuna and B. Hu for their technical assistance, and G. Warnock and Z. Ao from the Irving K. Barber Human Islet Isolation Laboratory (Vancouver, BC) for providing human islets.
Author information
Authors and Affiliations
Contributions
A. Rezania, J.E.B., J.D.J. and T.J.K. designed experiments, analyzed and interpreted results. J.E.B., P.A., A. Rubin, I.B., A.A., S.O., N.Q., M.M., T.A. and Y.H.C.Y. performed experiments. A. Rezania and T.J.K. conceived the project and supervised its participants. A. Rezania, J.E.B., J.D.J. and T.J.K. wrote the manuscript. A. Rezania, J.E.B., A.A., J.D.J., T.J.K., N.Q., M.M., T.A. and Y.H.C.Y. contributed to manuscript revisions.
Corresponding authors
Ethics declarations
Competing interests
A. Rezania, P.A., A. Rubin and I.B. are employees of Janssen R&D, LLC; T.J.K. received financial support from Janssen R&D, LLC, for the research described in this article.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–26 and Supplementary Tables 1–8 (PDF 16303 kb)
Rights and permissions
About this article
Cite this article
Rezania, A., Bruin, J., Arora, P. et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 32, 1121–1133 (2014). https://doi.org/10.1038/nbt.3033
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3033
This article is cited by
-
Metabolic switching, growth kinetics and cell yields in the scalable manufacture of stem cell-derived insulin-producing cells
Stem Cell Research & Therapy (2024)
-
Comparative and integrative single cell analysis reveals new insights into the transcriptional immaturity of stem cell-derived β cells
BMC Genomics (2024)
-
Current status of stem cell therapy for type 1 diabetes: a critique and a prospective consideration
Stem Cell Research & Therapy (2024)
-
Dual-targeted nano-encapsulation of neonatal porcine islet-like cell clusters with triiodothyronine-loaded bifunctional polymersomes
Discover Nano (2024)
-
ISR inhibition reverses pancreatic β-cell failure in Wolfram syndrome models
Cell Death & Differentiation (2024)