Abstract
Chromosomal instability is a hallmark of cancer that results from ongoing errors in chromosome segregation during mitosis. Although chromosomal instability is a major driver of tumour evolution, its role in metastasis has not been established. Here we show that chromosomal instability promotes metastasis by sustaining a tumour cell-autonomous response to cytosolic DNA. Errors in chromosome segregation create a preponderance of micronuclei whose rupture spills genomic DNA into the cytosol. This leads to the activation of the cGAS–STING (cyclic GMP-AMP synthase–stimulator of interferon genes) cytosolic DNA-sensing pathway and downstream noncanonical NF-κB signalling. Genetic suppression of chromosomal instability markedly delays metastasis even in highly aneuploid tumour models, whereas continuous chromosome segregation errors promote cellular invasion and metastasis in a STING-dependent manner. By subverting lethal epithelial responses to cytosolic DNA, chromosomally unstable tumour cells co-opt chronic activation of innate immune pathways to spread to distant organs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jamal-Hanjani, M. et al. Tracking the evolution of non-small-cell lung cancer. N. Engl. J. Med. 376, 2109–2121 (2017)
Turajlic, S. & Swanton, C. Metastasis as an evolutionary process. Science 352, 169–175 (2016)
Thompson, S. L. & Compton, D. A. Examining the link between chromosomal instability and aneuploidy in human cells. J. Cell Biol. 180, 665–672 (2008)
Cimini, D. et al. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J. Cell Biol. 153, 517–527 (2001)
Bakhoum, S. F. et al. The mitotic origin of chromosomal instability. Curr. Biol. 24, R148–R149 (2014)
Bakhoum, S. F. et al. Numerical chromosomal instability mediates susceptibility to radiation treatment. Nat. Commun. 6, 5990 (2015)
Bakhoum, S. F., Thompson, S. L., Manning, A. L. & Compton, D. A. Genome stability is ensured by temporal control of kinetochore–microtubule dynamics. Nat. Cell Biol. 11, 27–35 (2009)
Burrell, R. A. et al. Replication stress links structural and numerical cancer chromosomal instability. Nature 494, 492–496 (2013)
Brastianos, P. K. et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 5, 1164–1177 (2015)
Mitelman, F., Johansson, B. & Mertens, F. Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancerhttps://cgap.nci.nih.gov/Chromosomes/Mitelman (2017)
Chung, C. H. et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell 5, 489–500 (2004)
Moore, A. T. et al. MCAK associates with the tips of polymerizing microtubules. J. Cell Biol. 169, 391–397 (2005)
Ems-McClung, S. C. & Walczak, C. E. Kinesin-13s in mitosis: key players in the spatial and temporal organization of spindle microtubules. Semin. Cell Dev. Biol. 21, 276–282 (2010)
Waterman-Storer, C. M., Worthylake, R. A., Liu, B. P., Burridge, K. & Salmon, E. D. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat. Cell Biol. 1, 45–50 (1999)
Sotillo, R. et al. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell 11, 9–23 (2007)
Györffy, B. et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res. Treat. 123, 725–731 (2010)
Hatzis, C. et al. A genomic predictor of response and survival following taxane–anthracycline chemotherapy for invasive breast cancer. J. Am. Med. Assoc. 305, 1873–1881 (2011)
Abe, T. & Barber, G. N. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-κB activation through TBK1. J. Virol. 88, 5328–5341 (2014)
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP–AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013)
Hatch, E. M., Fischer, A. H., Deerinck, T. J. & Hetzer, M. W. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell 154, 47–60 (2013)
Ahn, J. et al. Inflammation-driven carcinogenesisis mediated through STING. Nat. Commun. 5, 5166 (2014)
Harding, S. M. et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 548, 466–470 (2017)
Mackenzie, K. J. et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 548, 461–465 (2017)
Lan, Y. Y., Londoño, D., Bouley, R., Rooney, M. S. & Hacohen, N. Dnase2a deficiency uncovers lysosomal clearance of damaged nuclear DNA via autophagy. Cell Reports 9, 180–192 (2014)
Denais, C. M. et al. Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–358 (2016)
Ly, P. et al. Selective Y centromere inactivation triggers chromosome shattering in micronuclei and repair by non-homologous end joining. Nat. Cell Biol. 19, 68–75 (2017)
Yang, H., Wang, H., Ren, J., Chen, Q. & Chen, Z. J. cGAS is essential for cellular senescence. Proc. Natl Acad. Sci. USA 114, E4612–E4620 (2017)
Stetson, D. B., Ko, J. S., Heidmann, T. & Medzhitov, R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134, 587–598 (2008)
Lau, L., Gray, E. E., Brunette, R. L. & Stetson, D. B. DNA tumor virus oncogenes antagonize the cGAS–STING DNA-sensing pathway. Science 350, 568–571 (2015)
Dou, Z. et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 550, 402–406 (2017)
Sun, S.-C. Controlling the fate of NIK: a central stage in noncanonical NF-κB signaling. Sci. Signal. 3, pe18 (2010)
Chen, Q. et al. Carcinoma–astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533, 493–498 (2016)
Wang, X. et al. Oestrogen signalling inhibits invasive phenotype by repressing RelB and its target BCL2. Nat. Cell Biol. 9, 470–478 (2007)
Wang, J., Yi, S., Zhou, J., Zhang, Y. & Guo, F. The NF-κB subunit RelB regulates the migration and invasion abilities and the radio-sensitivity of prostate cancer cells. Int. J. Oncol. 49, 381–392 (2016)
Yang, C. et al. Antagonism of inhibitor of apoptosis proteins increases bone metastasis via unexpected osteoclast activation. Cancer Discov. 3, 212–223 (2013)
Gulen, M. F. et al. Signalling strength determines proapoptotic functions of STING. Nat. Commun. 8, 427 (2017)
Kis-Toth, K., Szanto, A., Thai, T. H. & Tsokos, G. C. Cytosolic DNA-activated human dendritic cells are potent activators of the adaptive immune response. J. Immunol. 187, 1222–1234 (2011)
Sheltzer, J. M. et al. Single-chromosome gains commonly function as tumor suppressors. Cancer Cell 31, 240–255 (2017)
Weaver, B. A. A., Silk, A. D., Montagna, C., Verdier-Pinard, P. & Cleveland, D. W. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell 11, 25–36 (2007)
Rowald, K. et al. Negative selection and chromosome instability induced by Mad2 overexpression delay breast cancer but facilitate oncogene-independent outgrowth. Cell Reports 15, 2679–2691 (2016)
Robinson, D. R. et al. Integrative clinical genomics of metastatic cancer. Nature 548, 297–303 (2017)
Sheltzer, J. M. A transcriptional and metabolic signature of primary aneuploidy is present in chromosomally unstable cancer cells and informs clinical prognosis. Cancer Res. 73, 6401–6412 (2013)
McGranahan, N. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 (2016)
Bakhoum, S. F., Danilova, O. V., Kaur, P., Levy, N. B. & Compton, D. A. Chromosomal instability substantiates poor prognosis in patients with diffuse large B-cell lymphoma. Clin. Cancer Res. 17, 7704–7711 (2011)
Zaki, B. I., Suriawinata, A. A., Eastman, A. R., Garner, K. M. & Bakhoum, S. F. Chromosomal instability portends superior response of rectal adenocarcinoma to chemoradiation therapy. Cancer 120, 1733–1742 (2014)
Davidson, P. M., Denais, C., Bakshi, M. C. & Lammerding, J. Nuclear deformability constitutes a rate-limiting step during cell migration in 3-D environments. Cell. Mol. Bioeng. 7, 293–306 (2014)
Conway, T. et al. Xenome—a tool for classifying reads from xenograft samples. Bioinformatics 28, i172–i178 (2012)
Dobin, A . et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013)
Trapnell, C. et al. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010)
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015)
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014)
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005)
De Preter, K., Barriot, R., Speleman, F., Vandesompele, J. & Moreau, Y. Positional gene enrichment analysis of gene sets for high-resolution identification of overrepresented chromosomal regions. Nucleic Acids Res. 36, e43 (2008)
Gu, Z., Gu, L., Eils, R., Schlesner, M. & Brors, B. circlize implements and enhances circular visualization in R. Bioinformatics 30, 2811–2812 (2014)
Zheng, G. X. Y. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017)
van Dijk, D. et al. MAGIC: A diffusion-based imputation method reveals gene–gene interactions in single-cell RNA-sequencing data. Preprint at https://www.biorxiv.org/content/early/2017/02/25/111591 (2017)
Levine, J. H. et al. Data-driven phenotypic dissection of aml reveals progenitor-like cells that correlate with prognosis. Cell 162, 184–197 (2015)
Győrffy, B., Surowiak, P., Budczies, J. & Lánczky, A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS ONE 8, e82241 (2013)
Di Tommaso, P. et al. Nextflow enables reproducible computational workflows. Nat. Biotechnol. 35, 316–319 (2017)
Acknowledgements
We thank J. Massagué, R. Benezra, X. Cai, J. Leeman, M. Bakhoum, B. Hopkins and D. Landau for feedback. Grant support: S.F.B. (DoD Breast Cancer Research Breakthrough Award (BCRBA) W81XWH-16-1-0315, Elsa Pardee Foundation, MSKCC Cytogenetics Core (P30-CA008748) and Core (P30-CA008748) grants); B.N. (NSF Graduate Research Fellowship DGE1257284); J.L. (NIH R01-HL082792, U54-CA210184, DoD BCRBA BC150580, NSF CBET-1254846); P.L. (NCI K99-CA218871); G.G. (DoD BCRBA W81XWH-16-1-0316); L.C.C. (NIH R35-CA197588, U54-CA210184, Breast Cancer Research Foundation, Gray Foundation Basser Initiative).
Author information
Authors and Affiliations
Contributions
S.F.B. and L.C.C. conceived the project; S.F.B., B.N., J.-A.C. and R.K.S. performed animal experiments; V.K.R. derived PDX models; P.L. performed experiments using DLD-1 cells; S.F.B., B.N., J.A.C., R.K.S., M.D., S.V. and Q.L. performed immunostaining, immunoblotting, microscopy and qPCR; P.S. performed live-cell imaging; T.B.K.W., N.J.B. and N.M. analysed matched primary tumour–metastasis data; N.K.T., C.H.C. and S.F.B. analysed the HNSCC data; S.F.B. analysed breast cancer karyotype data; C.P. performed histological analysis; C.S., K.C. and G.N. performed cytogenetic analysis; A.M.L. analysed scRNSseq data; M.L. and S.F.B. analysed survival data; C.J.M. analysed bulk RNAseq data; M.I. analysed whole-genome sequence data; G.G., M.L., Q.L., J.H.H., O.E., C.H.C., N.Y.L., D.P., D.W.C., S.N.P., J.L., C.S. and L.C.C. assisted with data interpretation. All authors contributed to the writing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.C.C. owns equity in, receives compensation from, and serves on the board of directors and scientific advisory board of Agios Pharmaceuticals. He is also a founder of and receives laboratory support from Petra Pharmaceuticals. The other authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks N. Gekara, J. van Deursen and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Generation of isogenic tumour models of CIN.
a, wGII of brain metastases as a function of the wGII of the matched primary tumour. Red line represents linear regression, n = 79 patients. b, Differences in wGII between metastases and matched primary tumours. RCC, renal cell carcinoma; other includes melanoma, sarcoma, and ovarian, thyroid, and salivary gland cancers. c, Number of clones (based on single-cell karyotypes) in primary breast tumours (P; n = 637) or metastases (M; n = 131) found in the Mitelman database. Boxes represent median ± interquartile range and bars span the 10th and 90th percentiles; significance tested using two-sided Mann–Whitney test. d, The number of chromosome aberrations per clone as a function of the total number of chromosomes in a given clone in samples derived from primary breast tumour clones (n = 983) and metastatic clones (n = 186); data shown as mean ± s.d. e, Percentage of N− or N+ patients as a function of chromosome missegregation frequency (n = 20 patients per condition); significance tested using two-sided Fisher’s exact test. f, Immunoblots of cells expressing various GFP-tagged kinesin-13 proteins stained using anti-GFP antibody; β-actin used as a loading control, two independent experiments performed. g, Cellular confluence as a function of time in MDA-MB-231 cells expressing various kinesin-13 proteins or dnMCAK-expressing cells depleted of components of the cytosolic DNA-sensing machinery or the noncanonical NF-κB pathway. Data shown as mean ± s.d., n = 4 independent experiments. h, Left, MCAK- and dnMCAK-expressing cells stained for microtubules (α-tubulin, DM1A), centrosomes (pericentrin) and DNA (DAPI). Scale bar, 5 μm; two independent experiments performed. Right, frequency distribution of the number of pericentrin foci per cell. Significance tested using ANOVA. n = 100 cells per condition, two independent experiments performed. i, Chromosome missegregation in H2030 and 4T1 cells expressing kinesin-13 proteins. Data shown as mean ± s.d., n = 150 cells, three independent experiments performed, significance tested using two-sided t-test. j, Cells expressing kinesin-13 proteins stained for microtubules (DM1A), centrosomes (pericentrin) and DNA (DAPI). Scale bar, 50 μm, two independent experiments performed. k, Fluorescence normalized to cell count of MDA-MB0-231 cells expressing kinesin-13 proteins. Data shown as mean ± s.e.m., *P < 0.05, two-sided t-test, n = 10 high-power fields encompassing 477–612 cells, two independent experiments performed.
Extended Data Figure 2 Karyotype analyses of human tumour cells.
a, b, Immunoblots showing total RAC1 (a) or RHOA (b) levels as well as RAC1 or RHOA that was pulled down using antibodies that were specific to the GTP-bound form of RAC1 (a) or RHOA (b). Positive and negative controls were total MDA-MB-231 cell lysates supplanted with non-hydrolysable GTP (nhGTP) and GDP, respectively. β-actin was used as a loading control, two independent experiments performed. c–e, Representative karyotypes (DAPI-banding) from parental MDA-MB-231 cells (c) or populations derived from single cells expressing MCAK (d) or KIF2A (e) that were allowed to divide for 30 days. f, The number of non-clonal (present in less than 25% of the cells in a single clone) structurally abnormal chromosomes in CIN-low or CIN- high MDA-MB-231 cells. Mar, chromosomes so structurally abnormal that they could not be identified by conventional banding; data shown as mean ± s.d., n = 140 cells from 7 clonal populations, significance tested using two-way ANOVA test. g, Examples taken from four distinct cells belonging to the same clonal population (derived from a single KIF2A-expressing cell) showing convergent translocations involving chromosome 22 with four other chromosomes. h, Deviation from modal chromosome number in single-cell-derived clones grown for 30 days. Four chromosomes were assayed for each clone using centromere-specific probes. *P < 0.05, **P < 0.005 compared to control clone 4 by two-sided χ2-test, n = 300 cells per clone. Diploid controls were used to determine the false-positive rate of the centromeric probes.
Extended Data Figure 3 CIN promotes the formation and maintenance of metastasis.
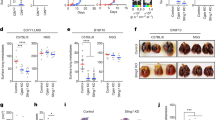
a, Normalized photon flux over time of whole animals injected with MDA-MB-231 cells expressing kinesin-13 proteins. Data shown as mean ± s.e.m. n = 8 (MCAK), 7 (KIF2B), 5 (control), 4 (KIF2A), and 9 (dnMCAK) mice per group; three independent experiments performed. b, Representative images of mice injected with MDA-MB-231 cells expressing dnMCAK (above) or KIF2B (below) with disease burden tracked using BLI; three independent experiments performed. c, Photon flux (p s−1) of whole animals imaged 5 weeks after intracardiac injection with control or MCAK-expressing H2030 cells. Horizontal bars represent the mean, significance tested using two-sided Mann–Whitney test, n = 10 mice in the MCAK group and 5 mice in the control group. d, Left, representative BLI images (from two independent experiments) of mice orthotopically transplanted with MDA-MB-231 cells. Images taken before (day 33) and after (day 90) tumour excision. Metastasis can be detected in the mouse transplanted with dnMCAK-expressing cells at day 90. Middle, total flux (p s−1) emitted from primary tumours 52 days after transplantation. Data shown as mean ± s.d., n = 5 (CIN-low) and 14 (CIN-high) mice, P = 0.13, two-sided Mann–Whitney test. Right, DMFS of mice orthotopically transplanted with MDA-MB-231 cells with various levels of CIN. n = 15 (CIN-low) and 29 (CIN-high) mice, pairwise significance tested with two-sided log-rank test. e, Tumour volume at 8 days (top) and survival (bottom) of mice transplanted with mouse 4T1 cells into the mammary fat pad. Bars represent median ± interquartile range, pairwise significance tested with two-sided t-test (top) and two-sided log-rank test (bottom). n = 20 (CIN-low) and 30 (CIN-high) mice. f, Top, immunoblots of MDA-MB-231 cells overexpressing MCAK or MCAK and MAD2 stained for MAD2 using anti-MAD2 antibody with α-tubulin used as a loading control; three independent experiments performed. Bottom, percentage of anaphase cells exhibiting evidence of chromosome missegregation in cells overexpressing MCAK or MCAK and MAD2. Data shown as mean ± s.d., n = 150 cells, three experiments performed, significance tested using two-sided t-test. g, Top, immunoblots of MDA-MB-231 cells overexpressing dnMCAK or dnMCAK and lamin B2 stained for lamin B2 using anti-lamin B2 antibody with β-actin used as a loading control. Two experiments performed. Bottom, percentage of anaphase cells exhibiting evidence of chromosome missegregation in cells overexpressing dnMCAK or dnMCAK and lamin B2. Data shown as mean ± s.d., n = 150 cells, three experiments performed, significance tested using two-sided t-test. h, Photon flux (p s−1) of whole animals after intracardiac (left) or tail vein (right) injection with MDA-MB-231 cells expressing dnMCAK or dnMCAK and lamin B2. Bars represent the median, significance tested using two-sided Mann–Whitney test, n = 9 (dnMCAK), 15 (dnMCAK and Lamin B2) mice in the intracardiac injection cohort and 5 mice per group in the tail vein injection cohort.
Extended Data Figure 4 Transcriptional consequences of CIN in cancer cells.
a, b, Principal component analysis (left) and unsupervised clustering (right) of five MDA-MB-231 cell lines expressing different kinesin-13 proteins based on bulk RNA expression data. b–e, Gene set enrichment analysis results showing HALLMARK gene sets that are highly enriched in CIN-high (control, KIF2A, and dnMCAK) compared with CIN-low cells (MCAK and KIF2B) (b, c) or STING-depleted cells (e), or after comparing metastases with primary tumours (d). Significance tested using one-sided weighted Smirnov–Kolmogorov test corrected for multiple tests. f, Heat map of consensus chromosomal karyotypes of cells derived from primary tumours and metastases showing selective increase in chromosome 1 copy number in metastases compared with primary tumours.
Extended Data Figure 5 Prognostic impact of CIN signature.
a, Volcano plot showing genes that were differentially expressed between CIN-high and CIN-low MDA-MB-231 cells. Red data points denote genes subsequently used for determining the CIN signature. b–e, Enrichment plots for all differentially expressed genes (a) or those on chromosome 1 (d, e). Circos plot (c) shows genomic location (outer circle), log2 fold expression of genes significantly differentially expressed in metastases compared to primary tumours (middle circles), and log10P (inner circle) for genomic amplifications (red) or deletions (blue) in metastases relative to primary tumours. n = 2 (CIN-low), 3 (CIN-high), 11 (primary tumours), 28 (metastases). Significance tested using two-sided Wald test (a), one-sided weighted Smirnov–Kolmogorov test (b, d, e), and one-sided hypergeometric test (c), all corrected for multiple testing. f, g, DMFS of breast cancer patients stratified by lymph node status, grade, and receptor status, from a meta-analysis (f, n = 664 patients) or a validation cohort (g, n = 171 patients) divided on the basis of average expression of the CIN gene expression. Significance tested using two-sided log-rank test.
Extended Data Figure 6 Single-cell sequencing and population detection.
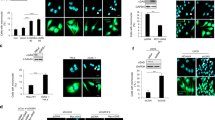
a, The cellular composition of every subpopulation presented Fig. 4b. b, Violin plots showing expression probability density of key metastasis and invasion genes in a subpopulation of cells (n = 1,273 cells) enriched for EMT and CIN genes (subpopulation M) compared with the remaining subpopulations (n = 5,548 cells) that were identified using graph-based unsupervised K-nearest neighbour embedding. c, Representative low-power field images (left) and numbers (right) of MDA-MB-231 cells that invaded through a collagen membrane within 18 h of culture. Data shown as mean ± s.d., significance tested using two-sided Mann–Whitney test, n = 10 high-power fields, two independent experiments performed. d, Representative images of MDA-MB-231 cells expressing MCAK or dnMCAK stained for β-actin, vimentin, and DNA. Scale bar, 50 μm, n = 2 independent experiments. e, Single-cell correlation plots between CIN signature genes, canonical NF-κB and type I interferon target genes, n = 6,821 cells. e, Representative phase-contrast images of a wound-healing assay of MDA-MB-231 cells expressing MCAK, MCAK and MAD2 or dnMCAK, and MCAK-expressing cells treated with cGAMP. Scale bar, 800 μm, four experiments performed.
Extended Data Figure 7 CIN promotes in vitro invasion and migration.
a, Left, representative phase-contrast images of MDA-MB-231 cells in the wound area, 36 h after wound creation. Four experiments performed. Right, length-to-width ratio of cells expressing different kinesin-13 proteins. Bars span the interquartile range, n = 100 cells, two independent experiments performed, significance tested using two-sided Mann–Whitney test. b, Representative MDA-MB-231 cells stained for β-catenin (anti-β-catenin antibody) or DNA (DAPI). Changes in β-catenin are seen upon alteration of CIN; it is enriched at cell–cell junctions in MCAK-expressing cells but is found in the cytoplasm and nucleus in dnMCAK-expressing cells. Scale bar, 30 μm, two experiments performed. c, Top, phase-contrast images of a wound-healing assay of cells expressing kinesin-13 proteins. Scale bar, 800 μm, two experiments performed. Bottom, wound area (normalized to the 0 h time point) 24 h and 45 h after wound creation. Data shown as mean ± s.d., n = 4 experiments, significance tested using two-sided t-test. d, Top, low-power field images of MDA-MB-231 cells that have migrated through a polycarbonate membrane containing 8-μm pores within 18 h of culture. Bottom, normalized OD of cells scraped from the bottom of the membrane. Data shown as mean ± s.e.m., significance tested using two-sided t-test, n = 3 experiments. e, f, Left, number of MDA-MB-231 cells that have successfully invaded through a collagen basement membrane 24 h after plating. Data shown as mean ± s.d., n = 20 high power fields from two independent experiments, significance tested using two-sided Mann–Whitney test. Right, representative images from high-power fields. Two independent experiments performed. g, i, Representative time-lapse fluorescence and phase-contrast image sequences of control cells expressing NLS–GFP undergoing unconfined migration (g) or going through 1 × 5-μm2 constrictions (i). Scale bars, 20 μm. Arrows in g indicate cytoplasmic NLS–GFP. Arrows in i indicate formation of nuclear protrusion and subsequent fragments during confined migration. Three independent experiments performed. h, j, Top, the probability of primary nuclear rupture during unconfined conditions (h) or after migration through 1 × 5-μm2 constrictions (j). Bottom, the number of cells migrating through more than one 1-μm-wide constrictions (j) and the duration of nuclear rupture (h), as measured by the length of time for which NLS–GFP signal is observed in the cytosol. Data shown as mean ± s.e.m., n = 3 independent experiments (except for unconfined rupture probability, 2 independent experiments) encompassing 390–665 (h) and 150–336 (j) cells observed during unconfined and confined migration, respectively. Significance tested using two-sided t-test.
Extended Data Figure 8 CIN generates micronuclei and cytosolic DNA.
a, b, Percentage of micronuclei in samples depicted in Fig. 3c, d: injected cells (blue), first-passage cells derived from primary tumours (green), or metastases (orange denotes spontaneous metastases arising from primary tumours, red denotes metastases obtained from direct intracardiac implantation). Data shown as mean ± s.e.m., n = 10 high-power fields encompassing 500–1,500 cells per sample, three independent experiments performed, *P < 0.05 (denotes samples with higher missegregation rates than the injected lines), #P < 0.05 (denotes samples with lower missegregation rates than the injected lines), **P < 0.05 (denotes significant differences between metastases and matched primary tumours from the same animals), two-tailed t-test. c, Correlation between the percentage of cells exhibiting evidence of chromosome missegregation and the percentage of micronuclei in all injected cell lines as well as cells derived from primary tumours and metastases. Data shown as mean ± s.e.m., n = 44 samples. d–f, Representative images of cells stained for DNA (DAPI), cytosolic single-stranded DNA (ssDNA) (d), DNASE2 (RFP reporter) (e), or cytosolic dsDNA (f). Scale bar, 20 μ μm, arrows in e denote DNASE2-expressing cell, two independent experiments performed. g, Representative images of dnMCAK-expressing cells treated with ssDNASE or dsDNASE for 10 min after selective plasma membrane permeabilization (using 0.02% saponin) and stained for DNA (DAPI) and cytosolic dsDNA. Scale bar, 20 μm, one experiment performed. h, Representative images of dnMCAK-expressing cells stained for mitochondria (anti-CoxIV antibody), DNA (DAPI) or for cytosolic DNA (anti-dsDNA antibody). Scale bar, 20 μm, two independent experiments performed. i, Immunoblots of lysates from cells expressing different kinesin-13 proteins, control or STING shRNA. β-actin used as a loading control. j, Normalized ratio of phosphorylated p52 to p100 (left) and p100 to total p100 (right) protein levels. Data shown as mean ± s.e.m., n = 5 independent experiments.
Extended Data Figure 9 Alternative response to cytosolic DNA in cancer cells.
a–d, Representative images of MDA-MB-231 cells stained for DNA (DAPI) and for p65 (a), IRF3 (b), or RELB (c, d). Images were individually contrast-enhanced to emphasize nuclear versus cytosolic localization of p65, IRF3, and RELB. For quantitative comparisons of identical images, see Supplementary Fig. 3. Arrows (c, d) point to RELB-positive nuclei. Scale bars, 20 μm, three independent experiments performed. e, Immunoblots of fractionated lysates. α-tubulin and lamin B2 were used as loading controls for the cytoplasmic and nuclear fractions, respectively; three independent experiments performed. f, h, Interferon-β levels in conditioned medium from DLD-1 cells (f), MDA-MB-231 or HEK293 cells with and without cGAMP addition (h). Data shown as mean ± s.e.m. n = 3 experiments, significance tested using one-sided Mann–Whitney test. g, i, Relative levels of interferon-responsive genes obtained by RT–qPCR in DLD-1 cells (g) normalized to untreated conditions or MDA-MB-231 cells (i) normalized to control cells. Data shown as mean ± s.d. n = 3 experiments, significance tested using two-sided t-test. j, Immunoblots of lysates of dnMCAK-expressing cells that also co-expressed control shRNA or shRNAs targeting components of the cytosolic DNA-sensing or noncanonical NF-κB pathways. shRNA hairpins are numbered in ascending order according to the efficiency of protein knockdown. Two independent experiments performed.
Extended Data Figure 10 Effect of cytosolic DNA-sensing pathways on prognosis.
a, Distant metastasis-free survival (DMFS), relapse-free survival (RFS) and progression-free survival (PFS) of patients with breast and lung, stratified according to their expression of NF-κB and interferon pathways. Significance tested using two-sided log-rank test. b, Disease-specific survival of mice injected with dnMCAK-expressing MDA-MB-231 cells co-expressing control shRNA, STING shRNA, NFKB2 shRNA, or RELB shRNA. n = 35, 16, 19, and 20 mice in the control, STING shRNA, NFKB2 shRNA, and RELB shRNA groups, respectively; significance tested using two-sided log-rank test. c, Number of MDA-MB-231 cells expressing shRNA targeting genes belonging to the DNA-sensing or noncanonical NF-κB pathways that invaded through a collagen membrane within 24 h of culture. Data shown as mean ± s.d., ** P < 0.0001, two-sided Mann–Whitney test, n = 20 high-power fields, two independent experiments performed. d, Number of different normal tissues (vascular, neuronal, or soft tissue) invaded by orthotopically transplanted tumours. Data shown as mean ± s.e.m., *P < 0.05, two-tailed t-test, n = 13 tumours (CIN-high), 20 tumours (noncanonical NF-κB depleted), 19 tumours (cGAS-STING depleted). e, Oncoprints showing genomic alterations in STING (TMEM173) and cGAS (MB21D1) in breast and lung cancers from the TCGA database.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-4 and Supplementary Figures 1-3 (PDF 4312 kb)
Supplementary Table 5
This table contains lists of genes belonging to the gene sets mentioned in the study. (XLSX 24 kb)
Supplementary Table 6
This table contains GSEA results. (XLSX 13 kb)
Source data
Rights and permissions
About this article
Cite this article
Bakhoum, S., Ngo, B., Laughney, A. et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 553, 467–472 (2018). https://doi.org/10.1038/nature25432
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature25432
This article is cited by
-
A benchmarking framework for the accurate and cost-effective detection of clinically-relevant structural variants for cancer target identification and diagnosis
Journal of Translational Medicine (2024)
-
Harnessing innate immune pathways for therapeutic advancement in cancer
Signal Transduction and Targeted Therapy (2024)
-
Second messenger 2'3'-cyclic GMP-AMP (2'3'-cGAMP): the cell autonomous and non-autonomous roles in cancer progression
Acta Pharmacologica Sinica (2024)
-
Scrambling the genome in cancer: causes and consequences of complex chromosome rearrangements
Nature Reviews Genetics (2024)
-
The two sides of chromosomal instability: drivers and brakes in cancer
Signal Transduction and Targeted Therapy (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.