Abstract
The canonical Wnt/β-catenin signalling pathway governs diverse developmental, homeostatic and pathological processes. Palmitoylated Wnt ligands engage cell-surface frizzled (FZD) receptors and LRP5 and LRP6 co-receptors, enabling β-catenin nuclear translocation and TCF/LEF-dependent gene transactivation1,2,3. Mutations in Wnt downstream signalling components have revealed diverse functions thought to be carried out by Wnt ligands themselves. However, redundancy between the 19 mammalian Wnt proteins and 10 FZD receptors1 and Wnt hydrophobicity have made it difficult to attribute these functions directly to Wnt ligands2,3. For example, individual mutations in Wnt ligands have not revealed homeostatic phenotypes in the intestinal epithelium4—an archetypal canonical, Wnt pathway-dependent, rapidly self-renewing tissue, the regeneration of which is fueled by proliferative crypt Lgr5+ intestinal stem cells (ISCs)5,6,7,8,9. R-spondin ligands (RSPO1–RSPO4) engage distinct LGR4–LGR6, RNF43 and ZNRF3 receptor classes10,11,12,13, markedly potentiate canonical Wnt/β-catenin signalling, and induce intestinal organoid growth in vitro and Lgr5+ ISCs in vivo8,14,15,16,17. However, the interchangeability, functional cooperation and relative contributions of Wnt versus RSPO ligands to in vivo canonical Wnt signalling and ISC biology remain unknown. Here we identify the functional roles of Wnt and RSPO ligands in the intestinal crypt stem-cell niche. We show that the default fate of Lgr5+ ISCs is to differentiate, unless both RSPO and Wnt ligands are present. However, gain-of-function studies using RSPO ligands and a new non-lipidated Wnt analogue reveal that these ligands have qualitatively distinct, non-interchangeable roles in ISCs. Wnt proteins are unable to induce Lgr5+ ISC self-renewal, but instead confer a basal competency by maintaining RSPO receptor expression that enables RSPO ligands to actively drive and specify the extent of stem-cell expansion. This functionally non-equivalent yet cooperative interaction between Wnt and RSPO ligands establishes a molecular precedent for regulation of mammalian stem cells by distinct priming and self-renewal factors, with broad implications for precise control of tissue regeneration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Clevers, H., Loh, K. M. & Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346, 1248012 (2014)
Willert, K. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 (2003)
Janda, C. Y., Waghray, D., Levin, A. M., Thomas, C. & Garcia, K. C. Structural basis of Wnt recognition by Frizzled. Science 337, 59–64 (2012)
Miyoshi, H., Ajima, R., Luo, C. T., Yamaguchi, T. P. & Stappenbeck, T. S. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science 338, 108–113 (2012)
Pinto, D., Gregorieff, A., Begthel, H. & Clevers, H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 17, 1709–1713 (2003)
Kuhnert, F. et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl Acad. Sci. USA 101, 266–271 (2004)
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007)
Yan, K. S. et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl Acad. Sci. USA 109, 466–471 (2012)
Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell 154, 274–284 (2013)
Xie, Y. et al. Interaction with both ZNRF3 and LGR4 is required for the signalling activity of R-spondin. EMBO Rep. 14, 1120–1126 (2013)
de Lau, W. et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 (2011)
Carmon, K. S., Gong, X., Lin, Q., Thomas, A. & Liu, Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc. Natl Acad. Sci. USA 108, 11452–11457 (2011)
Glinka, A. et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and Wnt/PCP signalling. EMBO Rep. 12, 1055–1061 (2011)
Kim, K. A. et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 309, 1256–1259 (2005)
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009)
Ootani, A. et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 15, 701–706 (2009)
Schuijers, J. et al. Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell 16, 158–170 (2015)
Hao, H. X. et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485, 195–200 (2012)
van der Flier, L. G., Haegebarth, A., Stange, D. E., van de Wetering, M. & Clevers, H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology 137, 15–17 (2009)
Tian, H. et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255–259 (2011)
Lopez-Garcia, C., Klein, A. M., Simons, B. D. & Winton, D. J. Intestinal stem cell replacement follows a pattern of neutral drift. Science 330, 822–825 (2010)
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010)
Peng, W. C. et al. Structure of stem cell growth factor R-spondin 1 in complex with the ectodomain of its receptor LGR5. Cell Rep. 3, 1885–1892 (2013)
Zebisch, M. et al. Structural and molecular basis of ZNRF3/RNF43 transmembrane ubiquitin ligase inhibition by the Wnt agonist R-spondin. Nat. Commun. 4, 2787 (2013)
Janda, C. Y. et al. Surrogate Wnt agonists that phenocopy canonical Wnt and/β-catenin signalling. Nature http://dx.doi.org/10.1038/nature22306 (this issue)
Kabiri, Z. et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 141, 2206–2215 (2014)
Hsieh, J. C., Rattner, A., Smallwood, P. M. & Nathans, J. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc. Natl Acad. Sci. USA 96, 3546–3551 (1999)
Koo, B. K. et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 488, 665–669 (2012)
Zheng, G. X. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017)
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015)
Grun, D. et al. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature 525, 251–255 (2015)
Storm, E. E. et al. Targeting PTPRK–RSPO3 colon tumours promotes differentiation and loss of stem-cell function. Nature 529, 97–100 (2016)
Wei, K. et al. A liver Hif-2α-Irs2 pathway sensitizes hepatic insulin signaling and is modulated by Vegf inhibition. Nat. Med. 19, 1331–1337 (2013)
Levin, T. G. et al. Characterization of the intestinal cancer stem cell marker CD166 in the human and mouse gastrointestinal tract. Gastroenterology 139, 2072–2082 (2010)
Proffitt, K. D. et al. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of Wnt-driven mammary cancer. Cancer Res. 73, 502–507 (2013)
Chao, G. et al. Isolating and engineering human antibodies using yeast surface display. Nat. Protocols 1, 755–768 (2006)
Trapnell, C., Pachter, L. & Salzberg, S. L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 (2009)
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015)
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014)
Trapnell, C. et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010)
Huang, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protocols 4, 44–57 (2009)
Yu, D., Huber, W. & Vitek, O. Shrinkage estimation of dispersion in Negative Binomial models for RNA-seq experiments with small sample size. Bioinformatics 29, 1275–1282 (2013)
McCarthy, D. J., Chen, Y. & Smyth, G. K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297 (2012)
Acknowledgements
We are indebted to members of the Kuo laboratory, G. Struhl, R. Nusse, L. O’Brien, D. Bouley, J. Carrington and H. Clevers for discussions, R. Nusse for WNT3A-expressing cells and FZD8 CRD cDNA and S. Robine for Villin-creER mice. We thank the Stanford Histology, Functional Genomics, Confocal, Neuroscience Imaging and Stanford Shared FACS Facility Cores. This work was supported by CIRM MD Scholar Fellowships (K.S.Y. and A.O), an AHA Postdoctoral Fellowship (J.C.), a Cancer Research Institute Irvington Fellowship (V.S.L.), Burroughs Wellcome Fund CAMS (K.S.Y.), NIH K08DK096048 (K.S.Y.), NIH K08DK100739 (A.H.), NIH K99CA204738 (V.S.L.), and the Ludwig Fund for Cancer Research (C.J.K., K.C.G.). C.J.K., P.D., R.J.F., S.J.H. and M.W. were supported by the Intestinal Stem Cell Consortium (NIH U01DK085527, U01DK085547, U01DK085525), which was funded by the NIDDK and NIAID. K.C.G. was supported by HHMI, the Stinehart/Reed Foundation, and NIH R01GM097015 and C.J.K. by an AHA Innovative Science Award and NIH U01CA176299, U19AI116484 and U01CA151920.
Author information
Authors and Affiliations
Contributions
K.S.Y. conceived, designed, and performed experiments, analysed data, and wrote the manuscript. J.C., J.Y. and A.J.H. participated in adenovirus cloning. K.A.L. and K.R. assisted with histology and in vivo experiments. C.Y.J., V.C.L. and K.C.G. contributed to biophysical studies. L.A.C., A.O., M.L., P.S.D, R.J.F., A.T.M., M.H.W. and S.J.H. assisted with characterization of LGR5 ECD and FZD8 CRD. H.U. and I.L.W. helped with clonal labelling experiments. C.R.B., J.W., X.L., A.H. and M.P.S. conducted bulk RNA-seq analysis. G.X.Y.Z., A.H., J.M.T., P.B., S.B.Z., H.O. and T.S.M. were involved in single-cell experiments. C.J.K. conceived and designed experiments, analysed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.Y.J., K.C.G. and C.J.K. are equity shareholders in Surrozen, Inc. G.X.Y.Z., J.M.T., P.B., S.B.Z., H.O. and T.S.M. are equity shareholders in 10x Genomics, Inc.
Additional information
Reviewer Information Nature thanks T. Sato and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
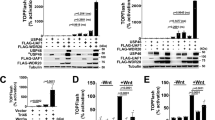
Extended Data Figure 1 Characterization of recombinant ectodomain proteins and adenoviruses for RSPO inhibition.
a, Recombinant LGR5 ECD, RNF43 ECD–Fc and ZNRF3 ECD–Fc proteins purified by Ni-NTA affinity chromatography, Coomassie-stained SDS–PAGE. b, Top, surface plasmon resonance analysis of LGR5 ECD binding to immobilized recombinant human RSPO1–RSPO4. Traces for RSPO1 are shown. Bottom, binding of RNF43 ECD–Fc or ZNRF3 ECD–Fc to mouse RSPO1–RSPO4 proteins. c, d, Recombinant LGR5-ECD inhibits recombinant human RSPO1 or recombinant mouse RSPO1–RSPO4 in a TOPflash Wnt reporter gene assay. Error bars represent s.e.m. *P < 0.05, unpaired Student’s t-test. e, Recombinant ZNRF3 ECD (mouse ZNRF3 ECD–Fc fusion) inhibits recombinant mouse RSPO1–RSPO4 in a TOPflash Wnt reporter assay. f, Recombinant RNF43 ECD (mouse RNF43 ECD–Fc fusion) inhibits recombinant mouse RSPO1–RSPO4 in TOPflash Wnt reporter assay. Error bars represent s.e.m. Low, med and high refer to 50:1, 250:1, 1,000:1 molar ratios of the respective recombinant ECD to the appropriate RSPO protein. *P < 0.05 versus the appropriate no ECD condition, unpaired Student’s t-test g, Time course of serum expression after a single intravenous injection of adenoviruses into C57Bl/6 mice. Top, anti-FLAG western blot was performed on serum at the indicated times after infection with Ad-LGR5-ECD (FLAG-tagged). Middle, time course of serum expression of ZNRF3 ECD–Fc fusion after single intravenous injection of Ad-Znrf3-ECD. Anti-IgG2α Fc western blot. Bottom, time course of serum expression of RNF43 ECD–Fc fusion after a single intravenous injection of Ad-Rnf43-ECD into C57Bl/6 mice. Anti-IgG2α Fc western blot. h, LGR5 ECD and ZNRF3 ECD bind simultaneously and non-exclusively to RSPO. Structure of RSPO1 (residues 40–132) highlighting distinct LGR5 ECD and ZNRF3/RNF43 ECD binding interfaces. i, Schematic of yeast display FACS experiment in which human RSPO2 is displayed on the extracellular surface via AGA2–Myc fusion. j–l, Unstained control for RSPO2 furin 1–2 domain-expressing yeast (j) or stained individually with 500 nM LGR5 ECD–FLAG-His or ZNRF3 ECD–Fc (k, l). m, n, Yeast were also stained sequentially with 500 nM LGR5 ECD–FLAG-His, washed, and then stained with a mixture of 500 nM ZNRF3 ECD–Fc plus 500 nM LGR5 ECD–FLAG-His to prevent competition or dissociation of LGR5 ECD–FLAG-His. ZNRF3 ECD–Fc was detected with Alexa Fluor 488-conjugated anti-mouse IgG. LGR5 ECD–FLAG-His was detected with Alexa Fluor 647-conjugated anti-FLAG (m). The order of this experiment was then reversed (ZNRF3 ECD–Fc first, then ZNRF3 ECD–Fc plus LGR5 ECD–FLAG-His) (n). The ability of the ZNRF3 and LGR5 ECDs to bind to RSPO2 simultaneously is shown by FACS staining in the top right quadrant (light green shading) (m, n).
Extended Data Figure 2 Time course of Ad-LGR5-ECD-induced ablation of Lgr5+ ISCs.
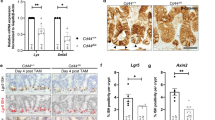
a, Lgr5–eGFP+ ISC signal is transiently lost from days 2–14 after single intravenous injection of Ad-LGR5-ECD, correlating with duration of transgenic overexpression of LGR5 ECD in sera of mice. Note that LGR5 ECD does not ablate the crypt compartment despite loss of Lgr5–eGFP+ ISC signal. b, Crypt-based Olfm4 expression is transiently lost from days 2–7 after single intravenous injection of Ad-LGR5-ECD. Olfm4 mRNA in situ hybridization. c, LGR5 ECD does not ablate crypt Ki67+ proliferation after Ad-LGR5-ECD despite loss of the Lgr5+ ISC signal. Scale bar, 50 μm. d, Higher magnification crypt images of Ki67+ cells after LGR5 ECD treatment. Scale bar, 20 μm. e, Quantification of d. Error bars represent s.e.m., P = 0.1215, unpaired Student’s t-test. f, Villus heights are not altered by LGR5 ECD. Error bars represent s.e.m., P = 0.2971, unpaired Student’s t-test. g, Strong suppression of Olfm4 in situ hybridization is observed on day 3 after treatment of mice with either Ad-Rnf43-ECD or Ad-Znrf3-ECD. Jejunum is shown.
Extended Data Figure 3 LGR5 ECD reduces ISCs/progenitors but not via apoptosis.
a, LGR5 ECD functionally reduces the number of ISCs/progenitors in neonatal mice. Multi-colour clonal labelling of intestinal epithelial cells in jejunum of neonatal Villin-creER; Rosa26-Rainbow mice, 8 days after tamoxifen induction resulting in stochastic clonal labelling to one of four fluorescent colours and 7 days after infection with Ad-LGR5-ECD compared to control Ad-Fc. Ad-LGR5-ECD induced premature crypt monoclonality, reflecting a functional decrease in the number of clones operating to repopulate the epithelium under conditions of RSPO inhibition, consistent with a marked reduction in ISC/progenitor number. Scale bars, 50 μm. b, LGR5 ECD does not induce apoptosis. TUNEL staining of jejunum at the indicated days after single intravenous injection of Ad-LGR5-ECD into mice reveals absence of crypt apoptosis. Positive control TUNEL staining after DNase I treatment of sections is also shown. Scale bar, 50 μm. c, FACS quantitation of yellow:red (Lgr5+ ISC:differentiated) cell ratio from Fig. 2a. Error bars represent s.e.m. d, FACS quantification of yellow:red (Lgr5+ ISC:differentiated) cell ratio from Fig. 2d, 4 days after treatment. Error bars represent s.e.m. e, Ad-Rspo1 expands both Lgr5+ ISCs and Lgr5− Ki67+ proliferative crypt cells consistent with ISC and transit amplifying cell expansion. Scale bar, 50 μm.
Extended Data Figure 4 Multi-lineage differentiation upon LGR5 ECD, RNF43 ECD or ZNRF3 ECD treatment.
a, Enterocyte, goblet and enteroendocrine differentiation are preserved upon LGR5 ECD, RNF43 ECD or ZNRF3 ECD treatment. Adult mice received a single intravenous injection of the indicated adenoviruses encoding soluble ECDs of LGR5, RNF43 (Fc fusion) or ZNRF3 (Fc fusion), or RSPO1 (Fc fusion), and the jejunum was analysed at day 7 after injection by H&E or staining with anti-FABP1 (enterocyte), PAS (goblet) or anti-CHGA (enteroendocrine). Multi-lineage differentiation was maintained. b, LGR5 ECD but not RNF43 ECD or ZNRF3 ECD induces Paneth cell loss (day 7 after infection). Scale bar, 50 μm. c, LGR5 ECD induces transient Paneth cell loss. Time course of lysozyme expression in jejunum after single intravenous injection of Ad-LGR5-ECD into mice. Scale bar, 50 μm. Note ballooning degeneration and upward migration of lysozyme+ Paneth cells at days 3–4 (yellow brackets), followed by near-total Paneth cell loss at days 7–10 and return of Paneth cells at day 14 after injection. The Paneth cell loss (after day 3) occurs after the loss of Lgr5–eGFP signal (day 2, Extended Data Fig. 2), and Paneth cell return correlates with the time course of disappearance of LGR5 ECD serum expression (Extended Data Fig. 1g). d, Quantitation of Paneth cell loss in small intestine, day 7, n = 3 animals per condition, Error bars represent s.e.m., *P < 0.05, unpaired Student’s t-test. e, Ad-LGR5-ECD induces loss of anti-CD166 immunofluorescence (CBC/Paneth marker), jejunum, day 7 after adenovirus treatment. f, H&E reveals ballooning degeneration and upward migration of lysozyme+ Paneth cells after Ad-LGR5-ECD treatment (yellow brackets and arrows), consistent with an intermediate cell phenotype. g, Electron microscopy analysis of intestinal crypts after Ad-LGR5-ECD intravenous injection reveals ballooning degeneration at days 3–4, followed by Paneth cell loss by day 7.
Extended Data Figure 5 Wnt analogue scFv–DKK1c functions via FZD receptors to support Lgr5+ ISCs and can substitute for endogenous Wnt proteins.
a–c, Dose-dependent effects of Ad-scFv–DKK1c with or without Ad-Rspo1 on the intestinal epithelium. a, H&E of jejunum on day 4 after adenovirus treatment. b, Mice were treated with adenovirus titres of 107 to 109 pfu. Ki67+ proliferation of jejunum on day 4 after adenovirus injection. c, Dose-dependent effects of scFv–DKK1c on Wnt target cyclin D1. Jejunum, immunofluorescence, day 4 after adenovirus injection. Scale bars, 100 μm. d, Wnt analogue scFv–DKK1c functionally substitutes for endogenous Wnt proteins in vivo to rescue Lgr5+ ISCs from C59-mediated loss. Loss of Lgr5–eGFP reporter signal (red box) by FACS analysis (left) and Olfm4 expression (right) from jejunum of mice treated with the small molecule PORCN inhibitor C59. Adenoviral overexpression of scFv–DKK1c prevents C59-mediated ablation of the reporter signal. Mice were treated with C59 for a total of 4 days that began 2 days after adenovirus injection. Scale bar, 100 μm. e, Wnt analogue scFv–DKK1c rescues in vivo phenotypes elicited by the Wnt antagonist FZD8 CRD. Ad-Fzd8-CRD-mediated loss of Olfm4 mRNA (top), Wnt target cyclin D1 immunohistochemistry (middle) and Ki67+ crypt proliferation (bottom) are rescued by concomitant adenoviral overexpression of scFv–DKK1c. Jejunum, day 4 after treatment with adenovirus. Scale bars, 50 μm.
Extended Data Figure 6 Functional characterization of scFv–DKK1c overexpression in vivo.
a, Crypt clonality in Actin-creER; Rosa-Rainbow mice on day 8 after tamoxifen to clonally label cells and day 7 after adenovirus injection. Recombinant adenovirus encoding either Fc, scFv–DKK1c or LGR5 ECD was administered as a single intravenous injection into mice. Left, immunofluorescence; red box indicates an example of crypt areas used for quantification. Scale bar, 50 μm. Right, quantification of crypt clonality. Crypt clonality is not altered by scFv–DKK1c versus Fc control (*P = 0.2854), whereas LGR5 ECD treatment quickly establishes crypt monoclonality (that is, single colour), **P < 0.0001, unpaired Student’s t-test. Error bars represent s.e.m. b, Lineage-tracing kinetics of scFv–DKK1c treatment, 2 days after simultaneous tamoxifen and adenovirus treatment. Scale bar, 50 μm. c, Paneth cell homeostasis is not perturbed by scFv–DKK1c overexpression. Days 4 and 7 after adenovirus treatment, jejunum. Scale bars, 50 μm. d, Wnt analogue scFv–DKK1c does not accelerate radiation injury-induced epithelial repair. H&E after 10 Gy total body irradiation injury. Jejunum. Mice were pre-treated with adenovirus encoding either Fc or scFv–DKK1c 2 days before 10 Gy irradation. Scale bar, 50 μm. e, Wnt analogue scFv–DKK1c does not expand Olfm4+ ISCs. Olfm4 in situ hybridization demonstrates lack of Olfm4 expansion upon Ad-scFv–DKK1c treatment compared to control and combinatorial treatment with RSPO1 does not expand Olfm4+ CBCs beyond the actions of RSPO1 alone. Jejunum, day 4 after treatment. Scale bar, 50 μm. f, Proliferative villus cells upon scFv-DKK1c plus RSPO1 treatment in Fig. 4a and Extended Data Fig. 6e do not express FABP1 by immunofluorescence staining.
Extended Data Figure 7 Comparative effects of scFv–DKK1c, WNT3A and scFV–DKK1c-RSPO2 adenoviruses on the intestinal epithelium.
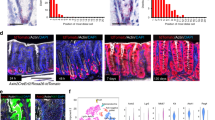
a–c, Mice were treated with adenovirus encoding either scFv–DKK1c or WNT3A with or without RSPO1/RSPO2 or scFv–DKK1c–RSPO2, and tissue was collected on day 4 after treatment. The single-chain polypeptide scFv–DKK1c–RSPO2 phenocopies combinatorial treatment with scFv–DKK1c plus RSPO. The effects of scFv–DKK1c–RSPO2 were present in a proximal-distal gradient and were confined to the proximal small intestine, in contrast to the effects of scFv–DKK1c plus RSPO1 or RSPO2, which were pervasive throughout the small intestine. a, H&E of jejunum on day 4 after treatment. b, Ki67 immunofluorescence. c, CD44 immunofluorescence. Scale bars, 100 μm (a–c). d, Lgr5-eGFP-IRES-creER; Rosa26-tdTomato mice were treated simultaneously with tamoxifen and intravenous adenovirus. Tissue was collected on day 4 after treatment. Notably, neither treatment with combined scFv–DKK1c plus RSPO2 nor the single-chain polypeptide scFv–DKK1–RSPO2 alters the lineage tracing of Lgr5+ ISCs compared to RSPO2 alone. Scale bar, 50 μm.
Extended Data Figure 8 Wnt analogue scFv–DKK1c does not substitute for RSPO loss in vivo.
a, Wnt analogue scFv–DKK1c restores crypts but not Olfm4 mRNA after dual ECD RSPO inhibition (LGR5 ECD plus ZNRF3 ECD). Adenoviral overexpression of scFv–DKK1c does not rescue combined LGR5 ECD- and ZNRF3 ECD-mediated loss of Olfm4 expression (top), despite reversing loss of the Wnt target gene cyclin D1 (middle) as well as Ki67+ crypt proliferation (bottom). Jejunum, day 4 after treatment. Scale bar, 100 μm. b, Wnt analogue scFv–DKK1c does not rescue Olfm4 expression after single ECD RSPO inhibition (LGR5-ECD). Olfm4 in situ hybridization, jejunum, day 4 after treatment with adenovirus. Scale bar, 100 μm.
Extended Data Figure 9 Single-cell RNA-seq, PCA and clustering.
a–f, Single-cell RNA-seq quality control metrics used to filter out cells before PCA and clustering. a, Distribution of the number of genes detected per cell. Cells with more than 400 genes, but less than 4,400 genes, were selected for PCA (red dashed lines). b, Distribution of number of unique molecular identifiers (UMIs) detected per cell. Cells with more than 861 UMIs, but less than 13,250 UMIs, were selected for PCA analysis (red dashed lines). c, Distribution of percentage of mitochondria UMIs per cell. Cells with less than 10% mitochondrial UMIs were selected for PCA analysis (red dashed lines). d, Number of genes and percentage of mitochondria UMIs detected per cell. e, Number of genes and UMIs detected per cell. f, Standard deviation of PC. g–h, Nine distinct clusters were detected among more than 13,000 single cells analysed by single cell RNA-seq. g, t-SNE projection of single cells, coloured by inferred cell type assignment. h, Normalized expression (centred) of the top variable genes (rows) from each of 9 clusters (columns) is shown in a heatmap. Numbers at the top indicate cluster number in g, with connecting lines indicating the hierarchical relationship between clusters. Gene symbols of markers from each cluster are shown on the right.
Extended Data Figure 10 Gene expression of additional marker genes in single-cell RNA-seq clusters upon Wnt versus RSPO modulation in vivo.
a, Reproduction of Fig. 5a for reference. b, t-SNE projection of more than 13,000 single cells divided over the 7 conditions, with each cell coloured based on their normalized expression of the indicated genes. UMI normalization was performed by first dividing UMI counts by the total UMI counts in each cell, followed by multiplication with the median of the total UMI counts across cells and calculation of the natural log of the UMI counts. Finally, each gene was normalized such that the mean signal for each gene is 0, and the standard deviation is 1. Note repression of CBC identity genes (Lgr5, Ascl2, Olfm4 and Rnf43) and Axin2 by LGR5 ECD and FZD8 CRD. Furthermore, Mki67 and Tuba1b are expressed in cycling Lgr5+ ISCs and transit amplifying cells but not non-cycling Lgr5+ ISCs at homeostasis (Fc), but are restricted only to transit amplifying cells after LGR5 ECD and FZD8 CRD. c, Similar t-SNE analysis of Fig. 5a for additional loci of interest (Lgr4, Tnfrsf19, Znrf3, Hist1h1b, Fzd2, Fzd7, Lrp5 and Lrp6). d, Normalized expression (centred) of the top variable genes (rows) of each sample (columns) against Fc control from Fig. 5a is shown in a heatmap. Sample numbers are shown at the top, with connecting lines indicating the hierarchical relationship between samples (based on the top variable genes identified). Gene symbols of markers from each cluster are shown on the right. ‘Fc_neg’ indicates the Lgr5–eGFP− population from an Ad-Fc-treated mouse.
Supplementary information
Supplementary Data
This zipped file contains Supplementary Tables 1-8 and a Supplementary table guide. (ZIP 18819 kb)
Rights and permissions
About this article
Cite this article
Yan, K., Janda, C., Chang, J. et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal. Nature 545, 238–242 (2017). https://doi.org/10.1038/nature22313
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature22313
This article is cited by
-
Human intestinal organoid-derived PDGFRα + mesenchymal stroma enables proliferation and maintenance of LGR4 + epithelial stem cells
Stem Cell Research & Therapy (2024)
-
Crosstalk between colorectal CSCs and immune cells in tumorigenesis, and strategies for targeting colorectal CSCs
Experimental Hematology & Oncology (2024)
-
Hypoxic preconditioning accelerates the healing of ischemic intestinal injury by activating HIF-1α/PPARα pathway-mediated fatty acid oxidation
Cell Death Discovery (2024)
-
Targeted activation of ferroptosis in colorectal cancer via LGR4 targeting overcomes acquired drug resistance
Nature Cancer (2024)
-
WAY-262611 ameliorates the inflammatory bowel disease by activating Wnt/β-catenin pathway
In Vitro Cellular & Developmental Biology - Animal (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.