Abstract
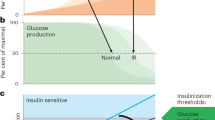
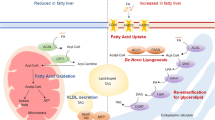
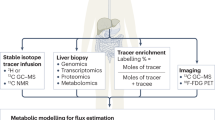
Non-alcoholic fatty liver disease and its downstream sequelae, hepatic insulin resistance and type 2 diabetes, are rapidly growing epidemics, which lead to increased morbidity and mortality rates, and soaring health-care costs. Developing interventions requires a comprehensive understanding of the mechanisms by which excess hepatic lipid develops and causes hepatic insulin resistance and type 2 diabetes. Proposed mechanisms implicate various lipid species, inflammatory signalling and other cellular modifications. Studies in mice and humans have elucidated a key role for hepatic diacylglycerol activation of protein kinase Cε in triggering hepatic insulin resistance. Therapeutic approaches based on this mechanism could alleviate the related epidemics of non-alcoholic fatty liver disease and type 2 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Browning, J. D. et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40, 1387–1395 (2004).
Smits, M. M., Ioannou, G. N., Boyko, E. J. & Utzschneider, K. M. Non-alcoholic fatty liver disease as an independent manifestation of the metabolic syndrome: results of a US national survey in three ethnic groups. J. Gastroenterol. Hepatol. 28, 664–670 (2013).
Fan, J.-G. et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J. Hepatol. 43, 508–514 (2005).
Amarapurkar, D. N. et al. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J. Gastroenterol. Hepatol. 22, 788–793 (2007).
Petersen, K. F. et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc. Natl Acad. Sci. USA 103, 18273–18277 (2006). This study reported ethnic differences in the prevalence of NAFLD and insulin resistance.
Das, K. et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology 51, 1593–1602 (2010).
Tolman, K. G., Fonseca, V., Dalpiaz, A. & Tan, M. H. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care 30, 734–743 (2007).
Wanless, I. R. & Lentz, J. S. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology 12, 1106–1110 (1990). This study reported that steatohepatitis was sevenfold more common in severely obese compared with patients of normal weight contributing to type 2 diabetes risk.
Silverman, J. F. et al. Liver pathology in morbidly obese patients with and without diabetes. Am. J. Gastroenterol. 85, 1349–1355 (1990).
Fabbrini, E., Sullivan, S. & Klein, S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 51, 679–689 (2010). This review summarizes the link between obesity, NAFLD and insulin resistance and the possible role of inflammation in these processes.
Shulman, G. I. Cellular mechanisms of insulin resistance. J. Clin. Invest. 106, 171–176 (2000). This review describes the cellular and molecular mechanisms of liver and muscle insulin resistance and proposes the diacylglycerol and novel PKC hypothesis of lipid-induced insulin resistance.
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Hui, J. M. et al. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology 38, 420–427 (2003).
Ratziu, V., Bellentani, S., Cortez-Pinto, H., Day, C. & Marchesini, G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol. 53, 372–384 (2010).
Charlton, M. R. et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 141, 1249–1253 (2011).
Samuel, V. T. & Shulman, G. I. Mechanisms for insulin resistance: common threads and missing links. Cell 148, 852–871 (2012). This review provides a balanced and detailed discussion of the potential roles of inflammation, ER stress, ceramides and other factors in the pathogenesis of liver and muscle insulin resistance.
Cheng, Z., Tseng, Y. & White, M. F. Insulin signaling meets mitochondria in metabolism. Trends Endocrinol. Metab. 21, 589–598 (2010).
Hanke, S. & Mann, M. The phosphotyrosine interactome of the insulin receptor family and its substrates IRS-1 and IRS-2. Mol. Cell. Proteomics 8, 519–534 (2009).
Franke, T. F., Kaplan, D. R., Cantley, L. C. & Toker, A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275, 665–668 (1997).
Adina-Zada, A. et al. Allosteric regulation of the biotin-dependent enzyme pyruvate carboxylase by acetyl-CoA. Biochem. Soc. Trans. 40, 567–572 (2012).
Adina-Zada, A., Zeczycki, T. N. & Attwood, P. V. Regulation of the structure and activity of pyruvate carboxylase by acetyl CoA. Arch. Biochem. Biophys. 519, 118–130 (2012).
Pilkis, S. J., el-Maghrabi, M. R. & Claus, T. H. Fructose-2,6-bisphosphate in control of hepatic gluconeogenesis. From metabolites to molecular genetics. Diabetes Care 13, 582–599 (1990).
Petersen, K. F., Laurent, D., Rothman, D. L., Cline, G. W. & Shulman, G. I. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. J. Clin. Invest. 101, 1203–1209 (1998).
Roden, M. et al. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Invest. 97, 2859–2865 (1996).
Samuel, V. T. et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 279, 32345–32353 (2004). The authors of this paper established a model of selective hepatic insulin resistance and demonstrated that this resistance was associated with increased hepatic diacylglycerol content and increased PKCε activation for the first time.
Samuel, V. T. et al. Inhibition of protein kinase Cε prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J. Clin. Invest. 117, 739–745 (2007). This paper conclusively demonstrated the key role of PKCε activation in mediating lipid-induced hepatic insulin resistance.
Dries, D. R., Gallegos, L. L. & Newton, A. C. A single residue in the C1 domain sensitizes novel protein kinase C isoforms to cellular diacylglycerol production. J. Biol. Chem. 282, 826–830 (2007).
Raddatz, K. et al. Time-dependent effects of Prkce deletion on glucose homeostasis and hepatic lipid metabolism on dietary lipid oversupply in mice. Diabetologia 54, 1447–1456 (2011).
Kumashiro, N. et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc. Natl Acad. Sci. USA 108, 16381–16385 (2011). This paper reports that intracellular diacylglycerol, associated with PKCε activation, is the strongest predictor of insulin resistance in obese patients.
Magkos, F. et al. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology 142, 1444–1446.e2 (2012).
Funke, A. et al. Cholesterol-induced hepatic inflammation does not contribute to the development of insulin resistance in male LDL receptor knockout mice. Atherosclerosis 232, 390–396 (2014).
Brown, M. S. & Goldstein, J. L. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7, 95–96 (2008).
Chavez, J. A. & Summers, S. A. Lipid oversupply, selective insulin resistance, and lipotoxicity: molecular mechanisms. Biochim. Biophys. Acta 1801, 252–265 (2010).
Kim, J. K. et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc. Natl Acad. Sci. USA 98, 7522–7527 (2001). This paper reports that overexpression of LpL in liver resulted in liver-specific triglyceride accumulation and liver-specific insulin resistance, whereas muscle-specific overexpression of LpL resulted in muscle-specific triglyceride accumulation and muscle-specific insulin resistance.
Doege, H. et al. Silencing of hepatic fatty acid transporter protein 5 in vivo reverses diet-induced non-alcoholic fatty liver disease and improves hyperglycemia. J. Biol. Chem. 283, 22186–22192 (2008).
Mittendorfer, B., Magkos, F., Fabbrini, E., Mohammed, B. S. & Klein, S. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity (Silver Spring) 17, 1872–1877 (2009).
Pardina, E. et al. Increased expression and activity of hepatic lipase in the liver of morbidly obese adult patients in relation to lipid content. Obes. Surg. 19, 894–904 (2009).
Weiss, R. et al. The 'obese insulin-sensitive' adolescent: importance of adiponectin and lipid partitioning. J. Clin. Endocrinol. Metab. 90, 3731–3737 (2005).
Cao, H. et al. Regulation of metabolic responses by adipocyte/macrophage Fatty Acid-binding proteins in leptin-deficient mice. Diabetes 55, 1915–1922 (2006).
Jaworski, K. et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nature Med. 15, 159–168 (2009).
Mingrone, G. et al. Triglyceride-induced diabetes associated with familial lipoprotein lipase deficiency. Diabetes 48, 1258–1263 (1999).
Auinger, A. et al. A promoter polymorphism in the liver-specific fatty acid transport protein 5 is associated with features of the metabolic syndrome and steatosis. Horm. Metab. Res. 42, 854–859 (2010).
Petersen, K. F. et al. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N. Engl. J. Med. 362, 1082–1089 (2010). The authors of this paper show that variants in APOC3 were associated with a high prevalence of NAFLD and insulin resistance in lean Asian-Indian men.
Peter, A., Kantartzis, K., Machicao, F. & Machann, J. Visceral obesity modulates the impact of apolipoprotein C3 gene variants on liver fat content. J. Obes. 36, 774–782 (2012). This paper, which follows up on the findings of ref. 43 shows that the association between APOC3 is only observable in lean, not obese individuals, demonstrating that obesity may mask the predisposing effects of APOC3 genetic variants on NAFLD and insulin resistance.
Camporez, J. P. G. et al. Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology 154, 1021–1028 (2013).
Verrijken, A., Beckers, S., Francque, S. & Hilden, H. A gene variant of PNPLA3, but not of APOC3, is associated with histological parameters of NAFLD in an obese population. Obesity (Silver Spring) 21, 2138–2145 (2013).
Kozlitina, J., Boerwinkle, E., Cohen, J. C. & Hobbs, H. H. Dissociation between APOC3 variants, hepatic triglyceride content and insulin resistance. Hepatology 53, 467–474 (2011).
Lee, H.-Y. et al. Apolipoprotein CIII overexpressing mice are predisposed to diet-induced hepatic steatosis and hepatic insulin resistance. Hepatology 54, 1650–1660 (2011). This study demonstrates that transgenic mice with hepatic overexpression of human APOC3 predisposes them to severe hepatic steatosis and hepatic insulin resistance when fed a high-fat diet, whereas there is no metabolic phenotype when they are fed a normal diet.
Kim, J. K., Gavrilova, O., Chen, Y., Reitman, M. L. & Shulman, G. I. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J. Biol. Chem. 275, 8456–8460 (2000). This study clearly illustrated the mechanism by which lipodystrophy syndromes lead to insulin resistance.
Wang, F., Mullican, S. E., DiSpirito, J. R., Peed, L. C. & Lazar, M. A. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARγ. Proc. Natl Acad. Sci. USA 110, 18656–18661 (2013).
Cortés, V. A. et al. Leptin ameliorates insulin resistance and hepatic steatosis in Agpat2−/− lipodystrophic mice independent of hepatocyte leptin receptors. J. Lipid Res. 55, 276–288 (2014).
Savage, D. B., Murgatroyd, P. R., Chatterjee, V. K. & O'Rahilly, S. Energy expenditure and adaptive responses to an acute hypercaloric fat load in humans with lipodystrophy. J. Clin. Endocrinol. Metab. 90, 1446–1452 (2005).
Petersen, K. F. et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Invest. 109, 1345–1350 (2002). This study established the mechanism by which leptin replacement therapy reverses liver and muscle insulin resistance in patients with lipodystrophy.
Simha, V., Szczepaniak, L. S., Wagner, A. J., DePaoli, A. M. & Garg, A. Effect of leptin replacement on intrahepatic and intramyocellular lipid content in patients with generalized lipodystrophy. Diabetes Care 26, 30–35 (2003).
Gandotra, S. et al. Perilipin deficiency and autosomal dominant partial lipodystrophy. N. Engl. J. Med. 364, 740–748 (2011).
Diraison, F., Moulin, P. & Beylot, M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 29, 478–485 (2003).
Petersen, K. F. et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc. Natl Acad. Sci. USA 104, 12587–12594 (2007). In this paper the authors demonstrate that selective insulin resistance in skeletal muscle promotes the development of atherogenic dyslipidaemia and NAFLD.
Donnelly, K. L. et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115, 1343–1351 (2005).
Petersen, K. F. et al. Reversal of muscle insulin resistance by weight reduction in young, lean, insulin-resistant offspring of parents with type 2 diabetes. Proc. Natl Acad. Sci. USA 109, 8236–8240 (2012). This study provides evidence that skeletal muscle insulin resistance in young lean insulin-resistant offspring of parents with type 2 diabetes can be attributed to increased intramyocellular lipid content.
Flannery, C., Dufour, S., Rabøl, R., Shulman, G. I. & Petersen, K. F. Skeletal muscle insulin resistance promotes increased hepatic de novo lipogenesis, hyperlipidemia, and hepatic steatosis in the elderly. Diabetes 61, 2711–2717 (2012).
Bilz, S. et al. Activation of the farnesoid X receptor improves lipid metabolism in combined hyperlipidemic hamsters. Am. J. Physiol. Endocrinol. Metab. 290, E716–E722 (2006).
Delarue, J., Normand, S., Couet, C. & Pachiaudi, C. Effects of free fatty acids on the metabolic response to oral fructose in lean healthy humans. Int. J. Obes. Relat. Metab. Disord. 20, 130–136 (1996).
Zhang, C. et al. Endoplasmic reticulum stress is involved in hepatic SREBP-1c activation and lipid accumulation in fructose-fed mice. Toxicol. Lett. 212, 229–240 (2012).
Lin, J. et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1β coactivation of SREBP. Cell 120, 261–273 (2005).
Nagai, Y. et al. The role of peroxisome proliferator-activated receptor γ coactivator-1 β in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 9, 252–264 (2009).
Savage, D. B. et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J. Clin. Invest. 116, 817–824 (2006). The authors of this study reported that decreasing hepatic expression of hepatic ACC1 and ACC2 in rats by ASO decreased hepatic lipogenesis and increased liver fat oxidation, resulting in protection from lipid-induced hepatic steatosis and hepatic insulin resistance.
Kim, J. K. et al. Glucose toxicity and the development of diabetes in mice with muscle-specific inactivation of GLUT4. J. Clin. Invest. 108, 153–160 (2001).
Wang, H. Y. et al. AS160 deficiency causes whole-body insulin resistance via composite effects in multiple tissues. Biochem. J. 449, 479–489 (2013).
Asai, A. et al. Dissociation of hepatic insulin resistance from susceptibility of non-alcoholic fatty liver disease induced by a high fat and high carbohydrate diet in mice. Am. J. Physiol. Gastrointest. Liver Physiol. http://dx.doi.org/10.1152/ajpgi.00291.2013 (2014).
Rabøl, R., Petersen, K. F., Dufour, S., Flannery, C. & Shulman, G. I. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals. Proc. Natl Acad. Sci. USA 108, 13705–13709 (2011). The authors of this paper demonstrate that reversal of muscle insulin resistance in healthy young lean insulin-resistant individuals with a single 45 minute bout of elliptical exercise reversed the abnormal pattern of energy distribution of energy storage following carbohydrate ingestion, thus offering strong evidence in support of a key role for selective muscle insulin resistance in promoting NAFLD and atherogenic dyslipidaemia as proposed in ref. 57.
Nagle, C. A. et al. Hepatic overexpression of glycerol-sn-3-phosphate acyltransferase 1 in rats causes insulin resistance. J. Biol. Chem. 282, 14807–14815 (2007).
Neschen, S., Morino, K., Hammond, L. E., Zhang, D. & Liu, Z. X. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA: glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab. 2, 55–65 (2005).
Yu, X. X. et al. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology 42, 362–371 (2005).
Choi, C. S. et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J. Biol. Chem. 282, 22678–22688 (2007).
Monetti, M. et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 6, 69–78 (2007).
Jornayvaz, F. R. et al. Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proc. Natl Acad. Sci. USA 108, 5748–5752 (2011).
Kantartzis, K. et al. The DGAT2 gene is a candidate for the dissociation between fatty liver and insulin resistance in humans. Clin. Sci. 116, 531–537 (2009).
Shindo, N. et al. Involvement of microsomal triglyceride transfer protein in nonalcoholic steatohepatitis in novel spontaneous mouse model. J. Hepatol. 52, 903–912 (2010).
Morán-Ramos, S. et al. Opuntia ficus indica (nopal) attenuates hepatic steatosis and oxidative stress in obese Zucker (fa/fa) rats. J. Nutr. 142, 1956–1963 (2012).
Singhal, N. S., Patel, R. T., Qi, Y., Lee, Y. S. & Ahima, R. S. Loss of resistin ameliorates hyperlipidemia and hepatic steatosis in leptin-deficient mice. Am. J. Physiol. Endocrinol. Metab. 295, E331–E338 (2008).
Neschen, S. et al. n-3 Fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-alpha-dependent manner. Diabetes 56, 1034–1041 (2007).
Zhang, D. et al. Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc. Natl Acad. Sci. USA 104, 17075–17080 (2007).
Houten, S. M. et al. Impaired amino acid metabolism contributes to fasting-induced hypoglycemia in fatty acid oxidation defects. Hum. Mol. Genet. 22, 5249–5261 (2013).
Jornayvaz, F. R. et al. A high fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am. J. Physiol. Endocrinol. Metab. 299, E808–E815 (2010).
Jornayvaz, F. R. et al. Thyroid hormone receptor-α gene knockout mice are protected from diet-induced hepatic insulin resistance. Endocrinology 153, 583–591 (2012).
Camporez, J. P. G. et al. Cellular mechanisms by which FGF21 improves insulin sensitivity in male mice. Endocrinology 154, 3099–3109 (2013).
Brown, W. H. et al. Fatty acid amide hydrolase ablation promotes ectopic lipid storage and insulin resistance due to centrally mediated hypothyroidism. Proc. Natl Acad. Sci. USA 109, 14966–14971 (2012).
Fullerton, M. D. et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nature Med. 19, 1649–1654 (2013).
Birkenfeld, A. L. et al. Deletion of the mammalian INDY homolog mimics aspects of dietary restriction and protects against adiposity and insulin resistance in mice. Cell Metab. 14, 184–195 (2011).
Sunny, N. E., Parks, E. J., Browning, J. D. & Burgess, S. C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 14, 804–810 (2011).
Satapati, S. et al. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J. Lipid Res. 53, 1080–1092 (2012).
Szendroedi, J. et al. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology 50, 1079–1086 (2009).
Schmid, A. I. et al. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care 34, 448–453 (2011).
Befroy, D. E. et al. Direct assessment of hepatic mitochondrial oxidative and anaplerotic fluxes in humans using dynamic 13C magnetic resonance spectroscopy. Nature Med. 20, 98–102 (2014).
Farese, R. V., Jr, Zechner, R., Newgard, C. B. & Walther, T. C. The problem of establishing relationships between hepatic steatosis and hepatic insulin resistance. Cell Metab. 15, 570–573 (2012).
Cohen, J. C., Horton, J. D. & Hobbs, H. H. Human fatty liver disease: old questions and new insights. Science 332, 1519–1523 (2011).
Sun, Z. & Lazar, M. A. Dissociating fatty liver and diabetes. Trends Endocrinol. Metab. 24, 4–12 (2013).
Niebergall, L. J., Jacobs, R. L., Chaba, T. & Vance, D. E. Phosphatidylcholine protects against steatosis in mice but not non-alcoholic steatohepatitis. Biochim. Biophys. Acta 1811, 1177–1185 (2011).
Jacobs, R. L. et al. Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J. Biol. Chem. 285, 22403–22413 (2010).
Xu, E. et al. Hepatocyte-specific Ptpn6 deletion promotes hepatic lipid accretion, but reduces NAFLD in diet-induced obesity: potential role of PPARγ. Hepatology 59, 1803–1815 (2013).
Ruiz, R. et al. Sterol regulatory element binding protein-1 (SREBP-1) is required to regulate glycogen synthesis and gluconeogenic gene expression in mouse liver. J. Biol. Chem. 289, 5510–5517 (2014).
Benhamed, F. et al. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J. Clin. Invest. 122, 2176–2194 (2012).
Brown, J. M. et al. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J. Lipid Res. 51, 3306–3315 (2010).
Cantley, J. L. et al. CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proc. Natl Acad. Sci. USA 110, 1869–1874 (2013). The authors of this study demonstrate the importance of compartmentation of DAG in modulating hepatic insulin resistance.
Bergman, B. C., Hunerdosse, D. M., Kerege, A., Playdon, M. C. & Perreault, L. Localisation and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia 55, 1140–1150 (2012).
Sun, Z. et al. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nature Med. 18, 934–942 (2012).
Kumashiro, N. et al. Role of patatin-like phospholipase domain-containing 3 on lipid-induced hepatic steatosis and insulin resistance in rats. Hepatology 57, 1763–1772 (2013).
Kumari, M. et al. Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell Metab. 15, 691–702 (2012).
Speliotes, E. K. et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 7, e1001324 (2011).
Lin, Y.-C., Chang, P.-F., Chang, M.-H. & Ni, Y.-H. Genetic variants in GCKR and PNPLA3 confer susceptibility to nonalcoholic fatty liver disease in obese individuals. Am. J. Clin. Nutr. 99, 869–874 (2014).
Petersen, K. F. et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54, 603–608 (2005). This study demonstrates that moderate (<10%) body weight reduction in obese patients with type 2 diabetes eating a 1,200 calorie hypocaloric diet corrected fasting plasma glucose concentrations, normalized rates of hepatic glucose reversed NAFLD and reversed hepatic insulin resistance independent of changes in circulating adipocytokines.
Lim, E. L. et al. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 54, 2506–2514 (2011).
Weiss, E. C., Galuska, D. A., Kettel Khan, L., Gillespie, C. & Serdula, M. K. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am. J. Prev. Med. 33, 34–40 (2007).
McGuire, M. T., Wing, R. R. & Hill, J. O. The prevalence of weight loss maintenance among American adults. Int. J. Obes. Relat. Metab. Disord. 23, 1314–1319 (1999).
Haufe, S. et al. Long-lasting improvements in liver fat and metabolism despite body weight regain after dietary weight loss. Diabetes Care 36, 3786–3792 (2013).
Mayerson, A. B., Hundal, R. S., Dufour, S. & Lebon, V. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes 51, 797–802 (2002). This study demonstrated that thiazolidinediones improve insulin sensitivity in patients with type 2 diabetes be decreasing hepatic steatosis and promoting a redistribution of fat to the subcutaneous fat compartment.
Kim, J. K. et al. Differential effects of rosiglitazone on skeletal muscle and liver insulin resistance in A-ZIP/F-1 fatless mice. Diabetes 52, 1311–1318 (2003).
Prieur, X. et al. Thiazolidinediones partially reverse the metabolic disturbances observed in Bscl2/seipin-deficient mice. Diabetologia 56, 1813–1825 (2013).
Dutchak, P. A. et al. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell 148, 556–567 (2012).
Miyazaki, Y. et al. Rosiglitazone improves downstream insulin receptor signaling in type 2 diabetic patients. Diabetes 52, 1943–1950 (2003).
Perry, R. J. et al. Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targeted mitochondrial uncoupler. Cell Metab. 18, 740–748 (2013). The authors of this article demonstrate that a liver-targeted mitochondrial uncoupling agent (DNP) resulted in around a 60% increase in hepatic fat oxidation, reductions in liver and muscle triglyceride and diacylglycerol content and reversal of liver and muscle insulin resistance in rat models of NAFLD and type 2 diabetes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perry, R., Samuel, V., Petersen, K. et al. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510, 84–91 (2014). https://doi.org/10.1038/nature13478
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13478
This article is cited by
-
The role of anti-diabetic drugs in NAFLD. Have we found the Holy Grail? A narrative review
European Journal of Clinical Pharmacology (2024)
-
Fatty acid synthesis suppresses dietary polyunsaturated fatty acid use
Nature Communications (2024)
-
Differential effects of plant-based flours on metabolic homeostasis and the gut microbiota in high-fat fed rats
Nutrition & Metabolism (2023)
-
The relationship between the ratio of gamma-glutamyltransferase to high-density lipoprotein cholesterol and the risk of diabetes mellitus using publicly available data: a secondary analysis based on a longitudinal study in Japan
Lipids in Health and Disease (2023)
-
Bmp8a deletion leads to obesity through regulation of lipid metabolism and adipocyte differentiation
Communications Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.