Abstract
Antigenic variation of the Plasmodium falciparum multicopy var gene family enables parasite evasion of immune destruction by host antibodies1,2. Expression of a particular var subgroup, termed upsA, is linked to the obstruction of blood vessels in the brain and to the pathogenesis of human cerebral malaria3,4,5,6. The mechanism determining upsA activation remains unknown. Here we show that an entirely new type of gene silencing mechanism involving an exonuclease-mediated degradation of nascent RNA controls the silencing of genes linked to severe malaria. We identify a novel chromatin-associated exoribonuclease, termed PfRNase II, that controls the silencing of upsA var genes by marking their transcription start site and intron-promoter regions leading to short-lived cryptic RNA. Parasites carrying a deficient PfRNase II gene produce full-length upsA var transcripts and intron-derived antisense long non-coding RNA. The presence of stable upsA var transcripts overcomes monoallelic expression, resulting in the simultaneous expression of both upsA and upsC type PfEMP1 proteins on the surface of individual infected red blood cells. In addition, we observe an inverse relationship between transcript levels of PfRNase II and upsA-type var genes in parasites from severe malaria patients, implying a crucial role of PfRNase II in severe malaria. Our results uncover a previously unknown type of post-transcriptional gene silencing mechanism in malaria parasites with repercussions for other organisms. Additionally, the identification of RNase II as a parasite protein controlling the expression of virulence genes involved in pathogenesis in patients with severe malaria may provide new strategies for reducing malaria mortality.
Similar content being viewed by others
Main
Beyond histone modifying enzymes7,8,9,10, additional post-transcriptional mechanisms may control antigenic variation of the P. falciparum multicopy var gene family. RNA processing and degradation in P. falciparum erythrocytic-stage parasites has been inadequately studied, and therefore its potential role in the post-transcriptional control of virulence genes is unknown. We examined the RNA exosome, a RNase complex involved in RNA processing and decay associated with RNA quality control in the nucleus and cytoplasm of eukaryotic cells11,12. Bioinformatic analysis predicts eight putative RNA exosome-associated proteins in the P. falciparum genome, including exoribonuclease functional domain-containing proteins Dis3 and Rrp6 (Extended Data Table 1) (PlasmoDB site at http://plasmodb.org). Here we identify a non-canonical exoribonuclease containing a putative RNase II domain with no further homology to known exosome subunits that regulates upsA var gene expression, designated PfRNase II (PlasmoDB accession number PF3D7_0906000).
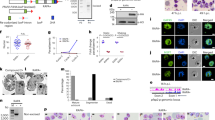
Structural prediction revealed homology between the RNase II domain of PfDis3 and human Dis3, and between PfRNase II and yeast Rrp44 (Extended Data Figs 1 and 2). By western blotting, antibodies against PfRNase II, PfDis3 and PfRrp6 reacted with bands of the predicted molecular masses 214, 135 and 126 kDa, respectively (Fig. 1a). To investigate the association between PfRNase II and core exosome complex, we generated a PfRrp4–haemagglutinin (HA)-tagged core exosome member transfectant and produced an antibody against another core exosome member, PfRrp45 (Extended Data Fig. 3a–d). Co-immunoprecipitation (CoIP) experiments showed that PfRNase II is a non-exosome exoribonuclease, whereas PfRrp6 and PfDis3 are the catalytic subunits of the plasmodial RNA exosome (Fig. 1b). Immunofluorescence assay (IFA) analysis revealed that PfRrp6 and PfRNase II localize as distinct foci at the nuclear periphery and that PfDis3 is localized in the cytoplasm adjacent to the nucleus at the ring stage (Fig. 1c). Immunoelectron microscopy confirmed the nuclear localization of PfRNase II (Extended Data Fig. 3e, f). To assess the RNA processing activity of the putative RNase II-like domain, we produced recombinant RNase II domains of PfRNase II and PfDis3 as glutathione S-transferase (GST) fusion proteins, using a PfRNase II-dead mutant and GST alone as negative controls (Fig. 1d and Extended Data Fig. 3g). In vitro RNA degradation assays revealed processive 3′–5′ hydrolytic activity towards single-stranded but not double-stranded RNA for PfRNase II and PfDis3, and no activity with the PfRNase II domain-dead mutant or GST alone (Fig. 1e and Extended Data Fig. 3h–j).
a, Schematic representation and western blot analysis of three plasmodial exosome exoribonuclease-like proteins PfRNase II, PfDis3 and PfRrp6. Proteins corresponding to the predicted molecular masses are indicated by arrows. IgG, preimmune antibody control; aa, amino-acid residues; Ab, antibody. b, CoIP assay of PfRrp4–HA (core exosome subunit) and PfRNase II–HA transfectants with various antibodies as indicated for each immunoprecipitation (IP) reaction. The anti-PfActin I antibody was used as control. c, Co-immunofluorescence assay of three plasmodial exoribonucleases and a core exosome member, PfRrp45, in ring-stage wild-type 3D7 parasites. The rabbit anti-PfRrp45, anti-PfRNase II, anti-PfRrp6 and mouse anti-PfDis3 antibodies were used in these assays. DAPI, 4′,6-diamidino-2-phenylindole. Scale bar, 1 μm. d, Schematic diagram of recombinant RNase II domain of PfRNase II and PfDis3. Dead mutant of PfRNase II catalytic domain and GST were used as negative controls. e, Exoribonuclease activity analysis of recombinant RNase II domains in vitro with single-stranded RNA probe (ssRNA). Data in a–c and e are representative of two independent experiments.
Attempts to disrupt PfRNase II in parasites by using conventional knockout strategies failed, indicating that this gene is essential for parasite growth (Extended Data Fig. 4a). We therefore engineered a ligand-regulated FK506-binding protein (FKBP) destabilization domain (DD) fusion protein to investigate PfRNase II protein knockdown in a parasite clone (3D7-G7) expressing a single upsC var gene, Pf3D7_0412700) (Fig. 2a). The DD fusion protein is stabilized by the synthetic ligand Shld1 and is rapidly degraded in its absence13. Three independent PfRNase II–FKBP clones G1, H3 and C3 were obtained (Extended Data Fig. 4b, c), and showed comparable propagation rates during the first two erythrocytic cycles. Without Shld1, however, PfRNase II–FKBP protein levels were decreased and parasites revealed drastic morphological changes in mature stages of the second cycle and were deficient in progressing to a third cycle (Fig. 2a and Extended Data Fig. 4d). To study the transcriptional role of PfRNase II, we used RNA-seq analysis on total RNA from synchronized ring-stage parasites 48 h after the removal of Shld1 (Extended Data Fig. 5a). Transcription patterns for the PfRNase II–FKBP clone H3 in the presence or absence of Shld1 were comparable except for 22 out of 37 mitochondrial genes with transcriptional upregulation (at least twofold) (Fig. 2b and Extended Data Fig. 5b), possibly explaining the observed lethal phenotype. Next, we compared transcriptional profiles of PfRNase II–FKBP parasites with those of wild-type parasites (3D7-G7). We observed more than fivefold upregulation in transcript levels for 213 genes in PfRNase II–FKBP in comparison with wild-type parasites (Fig. 2b, Extended Data Fig. 5c and Supplementary Table 1). This suggests that the carboxy-terminal FKBP tagging of PfRNase II disrupts interactions that are important for gene silencing or that it interferes with RNase II activity. Almost all transcriptional changes were observed for clonally variant virulence genes and non-coding RNA transcribed from regions adjacent to var genes. Strikingly, 14 upsA var genes are upregulated 5-fold to 270-fold, whereas most other subtypes (upsB and upsC) showed relatively minor changes. Moreover, two non-coding RNAs encoded by the 15-member family of GC-rich elements adjacent to internal var genes14 or from the subtelomeric non-coding TARE3 region showed strong upregulation in ring-stage parasites (Fig. 2c and Extended Data Fig. 5d). These data indicate the existence of a new type of post-transcriptional gene silencing of virulence genes.
a, Top: schematic diagram of PfRNase II–FKBP fusion protein. Bottom: growth curve of PfRNase II–FKBP clone H3 with or without Shld1. Parent 3D7-G7 was used as a control. Error bars represent s.e.m. for three independent experiments. b, Global transcriptome comparison of ring-stage parasite clones. Top: PfRNase II–FKBP Shld1+ versus Shld1−. Bottom: PfRNase II–FKBP Shld1+ versus wild-type 3D7-G7. Both axes are logarithmic and correspond to normalized read numbers of individual genes shown as dots. c, Statistical analysis of transcript level changes in PfRNase II–FKBP Shld1+ over 3D7-G7. The medians are indicated as a line, and whiskers illustrate the interquartile range. The cutoff (twofold) is indicated by a dashed line. Mito., mitochondrial. ***, P < 0.001; **, 0.001 < P < 0.01; *, 0.01 < P < 0.05 (two-tailed Student’s t-test). d, Transcriptional patterns of var gene family in ring-stage PfRNase II–FKBP-H3, G1, and C3 clones (Shld1+ or Shld1−) determined by qPCR. The five ups subtypes are indicated on the top. The data are shown as fold change of PfRNase II–FKBP over 3D7-G7 related to the seryl-tRNA synthetase gene as an internal control. e, Transcriptional profiles of intronic antisense long non-coding RNA (lncRNA) from three individual var genes with different ups-types in PfRNase II–FKBP clones compared with 3D7-G7 measured by qPCR. Data are represented as means ± s.e.m. for three independent experiments. f, Northern blot assay of 3D7-G7 and PfRNase II–FKBP-H3 and G1 clones. The probe corresponding to each clone was C1 var, A1 var and A2 var, respectively. The full-length transcript of each var gene is indicated by an arrow. Loading control for total RNA is shown by ethidium bromide staining. Data in a and f are representative of three independent experiments.
To validate the observed transcriptional changes in severe malaria-associated upsA var genes, we performed real-time quantitative PCR (qPCR) for individual var genes in parasite clones. Despite an increase in upsA var-type transcript levels in PfRNase II–FKBP transgenic parasites, only few members reached transcript levels observed during monoallelic expression. Up to three distinct upsA members were found to be co-transcribed at very high levels together with the upsC var C1 (3D7_0412700) expressed already in 3D7-G7 (Fig. 2d, f and Extended Data Fig. 6a). The upsA subgroup var genes are rarely activated in cultured parasites15,16. Each analysed clone expressed a different combination of upsA members, suggesting that switching occurs frequently in this subgroup, whereas upsC var C1 expression remained stable. Control experiments (3D7 parasites grown with Shld1 or harbouring a non-integrated FKBP plasmid) revealed a single dominant upsC but no upsA var gene (Extended Data Fig. 6b). To further demonstrate dissociation of the upsA var gene regulation, we cultured PfRNase II–FKBP parasite clones for a further 70 days (35 generations). qPCR analysis confirmed that most upsA var genes were upregulated in mutant clones but not in parent 3D7-G7 parasites, relative to day 0 (Extended Data Fig. 7a). For upsB and upsC var genes, some members showed higher transcript levels at day 70 but no striking differences between wild-type and mutant parasites. Subclone analysis of the C3 bulk culture (70 days) showed that new combinations of upsA var members co-transcribed with different members of upsC (Extended Data Fig. 6a). These data further illustrate the PfRNase II-dependent loss of monoallelic expression and continual switching of upsA var genes. In the PfRNase II–FKBP clones, a more than 60-fold transcriptional upregulation of antisense long non-coding RNA was observed from the upsA var gene intron region in ring-stage parasites, confirming the RNA sequencing result (Fig. 2e and Extended Data Fig. 8) and further highlighting a previously suggested role for exon 1 antisense long non-coding RNA in the process of var gene expression7,17,18.
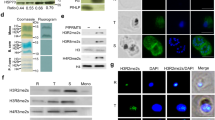
Single-cell analysis using two-colour RNA-fluorescence in situ hybridization (FISH) and surface immunofluorescence analysis confirmed the decoupling of upsA var genes from monoallelic expression in PfRNase II–FKBP parasites (Fig. 3a, b and Extended Data Fig. 7b). Using PfRNase II immunofluorescence with DNA-FISH (silent var) or RNA-FISH (active var A1), we observed a co-localization preference of PfRNase II protein to the silent var locus versus the active locus (Fig. 3c), indicating that removal of PfRNase II from the silent upsA var locus may trigger the gene activation in situ. We generated endogenous HA-tagged PfRNase II parasites to investigate its chromatin association by chromatin immunoprecipitation (ChIP)–qPCR, and confirmed that the C-terminal HA tag of PfRNase II did not interfere with monoallelic var expression (Extended Data Fig. 9a-d). ChIP analysis showed higher enrichment of PfRNase II protein at promoter and intron regions of upsA var genes, significantly less enrichment at other var gene types, and no association with the active one (Fig. 3d and Extended Data Fig. 9e). Given the potential role of PfRNase II in messenger RNA degradation, we performed nascent RNA analysis at var loci to study whether unstable mRNA was produced that was absent from steady-state RNA in wild-type 3D7-G7. We observed 15–20-fold higher levels of 5′ upsA var transcripts in nascent RNA than in total RNA. Non-upsA var genes and control genes showed slight, if any, difference between nascent and steady-state RNA levels (Fig. 4a), indicating cryptic mRNA production from upsA var genes. Further analysis revealed that both upstream region and intron promoter-derived antisense long non-coding RNA were the main sources of cryptic RNAs produced by upsA var genes (Fig. 4b and Extended Data Fig. 7c).
a, Two-colour RNA-FISH (left) and statistical analysis of co-localization (right) of var transcripts in PfRNase II–FKBP-H3 and G1 clones. Red, UpsC-type C1 var probe; green, upsA-type A1 var, A2 var and control gene probes (fructose-bisphosphate aldolase (FBA), PF3D7_1444800; Glutaminyl-tRNA synthetase (1370): PF3D7_1331700). Co., co-localization; adj., adjacent; non-co., non-co-localization. ***, P < 0.001 (χ2 test). Nuclear DNA was stained by DAPI (blue). Scale bar, 1 μm. Error bars represent s.e.m. for three independent experiments. b, Live-cell IFA using antisera to various PfEMP1s to detect co-expression of var genes in PfRNase II–FKBP H3 and G1 clones, respectively. The 3D7-G7 control expresses only one type of PfEMP1 (C1 var). Scale bar, 5 μm. c, PfRNase II is linked to the silent var-associated loci. Left: combined FISH/IFA assay using anti-PfRNase II antibody (IFA) with a FISH probe (PF3D7_0600400) that detected the upsA var expression site (RNA-FISH) in PfRNase II–FKBP clone H3. The same upsA var gene (DNA-FISH/IFA) was also analysed in ring-stage 3D7-G7 parasites. Right: statistical analysis of co-localization of each pair. In each analysis, more than 100 nuclei were counted; the error bars represent s.e.m. for three independent experiments. ***, P < 0.001 (χ2 test). d, ChIP–qPCR of PfRNase II–HA transfectant. The enrichment of representative var genes with anti-HA antibody were shown respectively. The qPCR probes for each var gene are indicated by short lines under var gene loci. Error bars represent s.e.m. for three independent experiments. The data in b are representative of three independent experiments. TARE3, telomere-associated repeat element 3.
a, Comparative qPCR var transcription analysis from nascent and steady-state mRNA in ring-stage 3D7-G7 wild-type parasites. Primers are designed against the upstream promoter regions of ups-specific or individual var and control genes. upsCs primers are silent upsC var-specific. The seryl-tRNA synthetase gene was used as an internal control for the calculation of relative copy numbers. Error bars represent s.e.m. for three independent experiments. b, Transcription profiles of nascent intronic antisense long non-coding RNA (lncRNA) from three individual var genes with different ups types from the steady-state RNA measured by qPCR assay. Transcription levels are shown as fold change of nascent RNA over total RNA. Error bars represent s.e.m. for three independent experiments. c, Transcription analysis of PfRNase II gene in field isolates with higher transcript levels of upsA var genes (from patients with severe malaria) and with lower levels (from patients with uncomplicated malaria). The LSA-1 and SSP2 genes with comparable transcriptional levels to those of PfRNase II are used as controls. **, P < 0.01 (two-tailed Student’s t-test).
To investigate the clinical relevance of our findings, we extracted total P. falciparum RNA from blood from patients with severe malaria and from patients with uncomplicated malaria. As observed previously19, upsA transcript levels were higher in patients with severe malaria than in those with uncomplicated malaria (Extended Data Fig. 7d), but PfRNase II transcripts were inversely correlated with upsA transcript levels (Fig. 4c), thereby linking decreased PfRNase II expression to upsA var gene regulation in patients with severe malaria.
Our findings identify a novel epigenetic silencing pathway in malaria parasites that is distinct from the post-transcriptional repression mechanism described previously20. Silenced upsA var genes continually produce short-lived sense and antisense transcripts from promoter and intron regions as a result of rapid in situ degradation by a novel type of RNase II. This may explain the several var transcripts detected in single infected erythrocytes (ring stage) by PCR21. By producing RNA from virtually all upsA var genes in RNase II-deficient parasites, singular expression of var genes is abolished. This raises the possibility that antisense long non-coding RNA is involved in the var promoter activation process. Our data show a resemblance to the degradation of cryptic unstable nuclear RNA by the exosome observed in yeast22. However, PfRNase II exerts its nuclear function dissociated from the RNA exosome core at specific chromatin regions (Extended Data Fig. 9f). We assume that the selective removal of PfRNase II at a specific upsA var locus allows expression of that locus, a notion supported by our co-localization experiments (Fig. 3c). Upregulation of other types of RNA in PfRNase II mutant parasites indicates that this type of mechanism might be a wide-ranging gene expression control mechanism.
In eukaryotic organisms, monoallelic expression controls distinct biological processes. For example in mice, only one of the ∼1,400 genes encoding olfactory receptors is expressed in any given olfactory sensory neuron23. Similarly, protozoan parasites that cause malaria, giardiasis or sleeping sickness express one of many possible variant surface antigen genes at a time24. This work identifies an entirely new mechanism contributing to monoallelic gene expression in malaria parasites that may be relevant in other organisms. In addition, the identification of the first Plasmodium protein controlling virulence factors expressed in patients with severe malaria may provide new strategies for reducing malaria pathogenesis.
Methods Summary
The association of exosome-like exoribonucleases and the core exosome complex was investigated by CoIP assays. FKBP destabilization domain and triple HA tagging at the C terminus of PfRNase II gene were carried out using the single-crossover recombinant strategy. The PfRNase II–FKBP clones were used in the comparative transcriptome analysis with wild-type 3D7-G7 clone by single-stranded RNA sequencing analysis25. The phenotypes of PfRNase II–FKBP clones were determined by RNA-FISH, IFA and fluorescence-activated cell sorting (FACS) with individual PfEMP1-specific antibodies in live infected red blood cells (iRBCs). The chromatin-associated PfRNase II was validated by electron microscopy, RNA-FISH, DNA-FISH and ChIP–qPCR analysis. We isolated the nascent RNA in a 3D7-G7 clone and used qPCR to measure the level of various regions of upsA var genes with other type var genes and housekeeping genes as controls, and compared them with those of steady-state RNA. All the primers used in this study are shown in Supplementary Table 2.
Online Methods
Plasmid constructs of transfection
Plasmid pLN-ENR-GFP26 was modified by replacement of pfenr-gfp with the 1.5-kilobase-pair C terminus of PfRNase II fused with the FKBP domain27 by ApaI/AflII. The resulting vector pLN-PfRNase II–FKBP was used to transfect 3D7 parasites. For the pLN-PfRNase II–HA construct, the FKBP fragment was replaced with sequence encoding the 3 × HA tag by AscI/AflII. For the PfRrp4–HA construct, the full-length coding region of PfRrp4 gene was cloned into the pLN vector by replacing the PfRNase II sequence of pLN–PfRNase II-HA construct with the PfRrp4 gene.
Parasite culture and transfection
P. falciparum 3D7 strain was cultivated and synchronized as described previously28. Ring-stage parasites at 5% parasitaemia were transfected with 100 μg of plasmid DNA by electroporation as described previously29. Transfected parasites were subjected to blasticidin S deaminase drug cycling in the presence of 0.5 μM Shld1 to select stable single-crossover parasites. After two cycles of three weeks off and two weeks on the drug, PfRNase II–FKBP or PfRNase II–HA integration events were detected in the transfected population analysed by PCR with genomic DNA with integrated PfRNase II locus-specific primers (Supplementary Table 2). To improve the selection efficiency of single-crossover clones, a third cycling was performed before limitation cloning.
Field isolates
In this study, patients who sought medical care and were diagnosed as having falciparum malaria at the local hospitals in Jiangsu Province in 2012 and 2013 were recruited with written informed consent; the clinical protocol was approved by the Internal Review Board of Jiangsu Institute of Parasitic Diseases (IRB00004221), China. Malaria diagnosis was done by microscopic examination of blood smears from patients. Severe malaria cases were defined according to the World Health Organization criteria for severe malaria30. Uncomplicated malaria was defined as the presence of asexual P. falciparum, an axillary temperature of more than 37.5 °C, or symptoms of headache but no other signs of severe malaria. The blood samples were collected from patients and lysed in Trizol (Invitrogen) directly, which were preserved at −80 °C until extraction of RNA. The transcription profiles of the var gene family in these collected samples were determined by qRT–PCR with the degenerate upsA-type specific primers31, and only those samples of severe malaria with high upsA-type var transcripts (7 out of 10), and uncomplicated malaria with silent upsA-type var genes (13 out of 13) were used for the comparative analysis of transcription levels of PfRNase II.
Quantitative reverse transcription PCR
qPCR assays were performed as described previously28. In brief, total RNA of synchronous parasite culture was extracted using Trizol reagent (Invitrogen)32. Complementary DNA was prepared in accordance with the manufacturer’s recommendations (Invitrogen). The fold change between the experimental and reference groups was calculated as  . The gene encoding seryl-tRNA synthetase (PF3D7_0717700) was used as the internal control. All qPCR primers used in this study are shown in Supplementary Table 2.
. The gene encoding seryl-tRNA synthetase (PF3D7_0717700) was used as the internal control. All qPCR primers used in this study are shown in Supplementary Table 2.
Production of recombinant exoribonucleases
The exoribonuclease catalytic domains of PfRNase II (residues 870–1321) and PfDis3 (residues 580–934) were amplified from cDNA (3D7) as template with the primers shown in Supplementary Table 2. The resulting PCR product was cloned into the vector pGEX-4T-1 by BamHI and EcoRI sites, followed by transformation into Escherichia coli BL21 (DE3) strain. Inducible expression of recombinant protein and affinity purification were performed in accordance with the manufacturer’s recommendations (GE Healthcare). The dead mutant of PfRNase II was generated by PCR mutagenesis.
In vitro exoribonuclease assay
The exoribonuclease reactions were performed as described33. In brief, the 17-mer oligoribonucleotides (5′-CCCCACCACCAUCACUU-3′) were labelled at their 5′ end with [γ-32P]ATP and T4 polynucleotide kinase (Promega). For the double-stranded oligoribonucleotides, labelled 17-mer oligonucleotide was hybridized to its complementary 17-mer oligoribonucleotides. The hybridization was performed in a 1:1 (mol/mol) ration in 10 mM Tris-HCl buffer pH 8.0 by incubation for 5 min at 68 °C followed by 1 h at 20 °C. The assays were performed in 12.5-μl reaction mixtures containing 20 mM Tris-HCl pH 8.0, 100 mM KCl and 1 mM MgCl2. The concentrations of recombinant RNase II protein and the substrate were 20 and 15 nM, respectively. Reactions were incubated at 37 °C for the indicated durations, and they were stopped by adding one volume of formamide-containing loading buffer (Ambion). Reaction products were resolved in a 20% (w/v) polyacrylamide, 7 M urea, and 1 × TBE gels and visualized by autoradiography.
Antibodies
Rabbit antibodies against PfRNase II, PfRrp6 and PfRrp45 were produced by GenScript Corporation (USA). The synthetic peptides (TIDPTKAKDLDDALC), (YHSKDEQIQTSKDE) and (NIRLDGRTFEDSRNC) conjugated with keyhole limpet haemocyanin were used to immunize rabbits in accordance with the standard protocols of GenScript. Mouse antibody against PfDIS3 was produced by the same strategy, and the synthetic peptide was (NNNKRPLYEPHLSKC).
Rabbit and rat antibodies against different 3D7 PfEMP1 DBL domains were generated against baculovirus recombinant proteins as described previously34.
Western blot analysis
Total parasite extracts were prepared by treatment with 0.15% saponin and resuspended in 1 × SDS-loading buffer (Bio-Rad), then separated on a 4–12% SDS–PAGE gel (Bio-Rad) and subjected to western blot analysis. The antibodies used in this study were rabbit anti-PfRNase II (dilution 1:500), rabbit anti-PfRrp6 (1:500), rabbit anti-PfRrp45 (1:500), mouse anti-PfDis3 (1:300), mouse anti-HA (1:2,000; Roche), rabbit anti-PfAldolase (1:1,000; Abcam) and rabbit anti-histone 3 (1:1,000; Abcam). Enhanced chemiluminescence (ECL) western blotting kit (GE healthcare) was used to develop blots.
Co-immunoprecipitation
Co-immunoprecipitation was performed as described previously, with some modifications35. In brief, parasite cultures were harvested and treated with 0.15% saponin. The released parasites were washed three times with 1 × PBS, then resuspended in six volumes of lysis buffer (25 mM Tris-HCl pH 7.5, 100 mM KCl, 2 mM EDTA, 0.5 mM phenylmethylsulphonyl fluoride, 0.05% Nonidet P40, 1 × protease inhibitor cocktail (Thermo)) and subjected to sonication on the highest power setting for 3 min at 30-s intervals with a sonicator (Bioruptor). The supernatants of the lysates were isolated and immediately incubated at 4 °C for 2 h with magnetic beads (Thermo) pre-coupled with various antibodies. After the beads had been washed twice with IPP500 (500 mM NaCl, 10 mM Tris-HCl pH 8.0, 0.05% Nonidet P40) and once with 1 × PBS, bound proteins were eluted with sample buffer for SDS–PAGE and western blot analysis.
Chromatin immunoprecipitation (ChIP)
The ChIP assay was performed as described previously36. In brief, highly synchronized ring-stage PfRNase II–HA parasites were cross-linked for 10 min with 1% paraformaldehyde and released from iRBC with 0.15% saponin lysis. Nuclei were isolated by Dounce homogenization, and chromatin was sheared into 200–500-base-pair fragments with a sonicator (Bioruptor) on the highest power setting for 10 min at 30-s intervals. Each 250-μl chromatin aliquot was incubated with 1 μg of mouse antibody against HA tag (Roche) or mouse IgG (Sigma) as control. The eluted DNAs were analysed by qPCR with the primers listed in Supplementary Table 2.
Nascent RNA-qPCR assay
The labelling of nascent RNA in P. falciparum was performed as described37. In brief, highly synchronized ring-stage parasites (1 ml) were permeabilized by 0.02% saponin, then subjected to transcription in live cells with incorporation of 5-ethynyl uridine (Invitrogen). The labelling reaction was performed at 37 °C for 20 min, the total RNA was isolated by Trizol, and the nascent RNA was further conjugated with biotin to be affinity-purified by streptavidin-coupled beads in accordance with the manual of the Click-iT Nascent RNA Capture Kit (Invitrogen). The purified nascent RNA was reverse-transcribed into cDNA with random primers for mRNA, or with 3ex1 specific primer for the intronic antisense non-coding RNA of var genes7. The qPCR assay of var genes and control genes was performed as for total extracted RNA.
Immunoelectron microscopy
Immunolabelling for electron microscopy was performed on ultrathin sections of glutaraldehyde-fixed synchronous parasites as described previously28. Rabbit anti-PfRNase II (1/50) and 10-nm protein A-colloidal gold was performed in accordance with the same protocol.
Northern blot
Northern blot analyses of individual full-length var genes in PfRNase II–FKBP clones and 3D7-G7 were performed as described previously38. The A1, A2 and C1 var probe templates were amplified from 3D7 genomic DNA with the primers listed in Supplementary Table 2.
Immunofluorescence and FISH
Immunofluorescence assay and DNA/RNA-FISH were performed as described previously8. The antibody dilution for rabbit anti-PfRNase II was 1:300, that for mouse anti-PfDIS3 was 1:100 and that for anti-PfRrp45 was 1:800; second antibodies of Alexa-Fluor-488 (or 568)-conjugated anti-rabbit or anti-mouse IgG were diluted 1:2,000. For immunostaining combining two rabbit primary antibodies, the Zenon labelling kit (Invitrogen) in accordance with the manufacturer’s instructions. For the individual var gene-specific RNA-FISH probes, DNA templates were amplified with the primers shown in Supplementary Table 2 and used to probe preparation with a Biotin- or Fluorescein-High Prime kit (Roche).
Flow cytometry
The generation of individual PfEMP1-specific antibodies and the detection of PfEMP1 expression on single parasite-infected blood cells by IFA and FACS had been described previously34. In brief, all samples analysed by flow cytometry were preliminarily detected by indirect immunofluorescence. For each sample, after purification with plasmion, 5 × 107 red blood cells infected with mature-stage 3D7 parasites were incubated with individual antibodies (dilution 1:20) or in combination with various antibodies as indicated in the text. The nuclei were stained with DAPI (Invitrogen).
RNA-seq analysis
The RNA isolation, mRNA enrichment and library construction were described previously25. In brief, highly synchronous parasites were harvested at the ring stage (10 h after invasion), and total RNAs were isolated with a miRNeasy Mini Kit (Qiagen). mRNA was enriched by poly(A) selection with a Dynabeads mRNA purification kit (Invitrogen), then fragmented to about 100–200 nucleotides (nt) in length, and treated for 2 h with 10 U of tobacco acid pyrophosphatase (Epicentre) at 37 °C to remove the 5′-terminal 7-methylguanylate cap. All subsequent steps were performed in accordance with an Illumina application note, and the last high-throughput sequencing was performed on a HiSeq2000 (Illumina).
Alignment of sequence tags and determination of transcript levels
To ensure comparable alignment between sequence reads from different sequencing runs, all sequenced cDNA reads were trimmed to a uniform length of 46 nt (4 nt were trimmed at the 5′ end and 50 or 0 nt at the 3′ end, depending on sequence read length). The trimmed sequence reads were aligned to the P. falciparum genome (version 7.1) using the bowtie alignment algorithm39 and allowing not more than two mismatches. Coding and non-coding RNA transcript levels were calculated as the number of reads per kilobase per million reads mapped (RPKM)40. To control for variations in mRNA enrichment, only sequence reads not aligning to ribosomal DNA were considered. For comparisons between the three cell lines used in this study, unique and non-uniquely aligned sequence reads were counted; for comparisons within a particular cell line, only uniquely aligning reads were considered. For the calculation of transcription level changes we used a cutoff RPKM > 0.5 in the analysis as described recently41.
Accession codes
Primary accessions
European Nucleotide Archive
Data deposits
The RNA-seq data generated for this study have been deposited in the European Nucleotide Archive under accession number PRJEB4511, and RNA-seq data from wild-type 3D7 cells25 used for this study are available under accession no. ERP001849.
References
Miller, L. H., Baruch, D. I., Marsh, K. & Doumbo, O. K. The pathogenic basis of malaria. Nature 415, 673–679 (2002)
Scherf, A., Riviere, L. & Lopez-Rubio, J. J. SnapShot: var gene expression in the malaria parasite. Cell 134, 190–190.e1 (2008)
Deitsch, K. W. & Chitnis, C. E. Molecular basis of severe malaria. Proc. Natl Acad. Sci. USA 109, 10130–10131 (2012)
Avril, M. et al. A restricted subset of var genes mediates adherence of Plasmodium falciparum-infected erythrocytes to brain endothelial cells. Proc. Natl Acad. Sci. USA 109, E1782–E1790 (2012)
Claessens, A. et al. A subset of group A-like var genes encodes the malaria parasite ligands for binding to human brain endothelial cells. Proc. Natl Acad. Sci. USA 109, E1772–E1781 (2012)
Turner, L. et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 498, 502–505 (2013)
Jiang, L. et al. PfSETvs methylation of histone H3K36 represses virulence genes in Plasmodium falciparum. Nature 499, 223–227 (2013)
Lopez-Rubio, J. J., Mancio-Silva, L. & Scherf, A. Genome-wide analysis of heterochromatin associates clonally variant gene regulation with perinuclear repressive centers in malaria parasites. Cell Host Microbe 5, 179–190 (2009)
Freitas-Junior, L. H. et al. Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell 121, 25–36 (2005)
Duraisingh, M. T. et al. Heterochromatin silencing and locus repositioning linked to regulation of virulence genes in Plasmodium falciparum. Cell 121, 13–24 (2005)
Jacquier, A. The complex eukaryotic transcriptome: unexpected pervasive transcription and novel small RNAs. Nature Rev. Genet. 10, 833–844 (2009)
Kiss, D. L. & Andrulis, E. D. The exozyme model: a continuum of functionally distinct complexes. RNA 17, 1–13 (2011)
Armstrong, C. M. & Goldberg, D. E. An FKBP destabilization domain modulates protein levels in Plasmodium falciparum. Nature Methods 4, 1007–1009 (2007)
Mourier, T. et al. Genome-wide discovery and verification of novel structured RNAs in Plasmodium falciparum. Genome Res. 18, 281–292 (2008)
Frank, M., Dzikowski, R., Amulic, B. & Deitsch, K. Variable switching rates of malaria virulence genes are associated with chromosomal position. Mol. Microbiol. 64, 1486–1498 (2007)
Horrocks, P., Pinches, R., Christodoulou, Z., Kyes, S. A. & Newbold, C. I. Variable var transition rates underlie antigenic variation in malaria. Proc. Natl Acad. Sci. USA 101, 11129–11134 (2004)
Ralph, S. A. et al. Transcriptome analysis of antigenic variation in Plasmodium falciparum-var silencing is not dependent on antisense RNA. Genome Biol. 6, R93 (2005)
Epp, C., Li, F., Howitt, C. A., Chookajorn, T. & Deitsch, K. W. Chromatin associated sense and antisense noncoding RNAs are transcribed from the var gene family of virulence genes of the malaria parasite Plasmodium falciparum. RNA 15, 116–127 (2009)
Jensen, A. T. et al. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J. Exp. Med. 199, 1179–1190 (2004)
Mair, G. R. et al. Regulation of sexual development of Plasmodium by translational repression. Science 313, 667–669 (2006)
Chen, Q. et al. Developmental selection of var gene expression in Plasmodium falciparum. Nature 394, 392–395 (1998)
Gudipati, R. K., Neil, H., Feuerbach, F., Malabat, C. & Jacquier, A. The yeast RPL9B gene is regulated by modulation between two modes of transcription termination. EMBO J. 31, 2427–2437 (2012)
Chess, A., Simon, I., Cedar, H. & Axel, R. Allelic inactivation regulates olfactory receptor gene expression. Cell 78, 823–834 (1994)
Deitsch, K. W., Lukehart, S. A. & Stringer, J. R. Common strategies for antigenic variation by bacterial, fungal and protozoan pathogens. Nature Rev. Microbiol. 7, 493–503 (2009)
Siegel, T. N. et al. Strand-specific RNA-Seq reveals widespread and developmentally regulated transcription of natural antisense transcripts in Plasmodium falciparum. BMC Genomics 15, 150 (2014)
Nkrumah, L. J. et al. Efficient site-specific integration in Plasmodium falciparum chromosomes mediated by mycobacteriophage Bxb1 integrase. Nature Methods 3, 615–621 (2006)
Armstrong, C. M. & Goldberg, D. E. An FKBP destabilization domain modulates protein levels in Plasmodium falciparum. Nature Methods 4, 1007–1009 (2007)
Zhang, Q. et al. A critical role of perinuclear filamentous actin in spatial repositioning and mutually exclusive expression of virulence genes in malaria parasites. Cell Host Microbe 10, 451–463 (2011)
Fidock, D. A. & Wellems, T. E. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc. Natl Acad. Sci. USA 94, 10931–10936 (1997)
Baker J. R., ed. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94 (Suppl. 1). S1–S90 (2000)
Rottmann, M. et al. Differential expression of var gene groups is associated with morbidity caused by Plasmodium falciparum infection in Tanzanian children. Infect. Immun. 74, 3904–3911 (2006)
Kyes, S., Pinches, R. & Newbold, C. A simple RNA analysis method shows var and rif multigene family expression patterns in Plasmodium falciparum. Mol. Biochem. Parasitol. 105, 311–315 (2000)
Amblar, M., Barbas, A., Fialho, A. M. & Arraiano, C. M. Characterization of the functional domains of Escherichia coli RNase II. J. Mol. Biol. 360, 921–933 (2006)
Joergensen, L. et al. Surface co-expression of two different PfEMP1 antigens on single Plasmodium falciparum-infected erythrocytes facilitates binding to ICAM1 and PECAM1. PLoS Pathog. 6, e1001083 (2010)
Staals, R. H. et al. Dis3-like 1: a novel exoribonuclease associated with the human exosome. EMBO J. 29, 2358–2367 (2010)
Lopez-Rubio, J. J., Mancio-Silva, L. & Scherf, A. Genome-wide analysis of heterochromatin associates clonally variant gene regulation with perinuclear repressive centers in malaria parasites. Cell Host Microbe 5, 179–190 (2009)
Mancio-Silva, L. & Scherf, A. In situ fluorescence visualization of transcription sites and genomic loci in blood stages of Plasmodium falciparum. Methods Mol. Biol. 923, 335–351 (2013)
Kyes, S. A. et al. A well-conserved Plasmodium falciparum var gene shows an unusual stage-specific transcript pattern. Mol. Microbiol. 48, 1339–1348 (2003)
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009)
Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature Methods 5, 621–628 (2008)
Xue, Z. et al. Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature 500, 593–597 (2013)
Schaeffer, D. et al. The exosome contains domains with specific endoribonuclease, exoribonuclease and cytoplasmic mRNA decay activities. Nature Struct. Mol. Biol. 16, 56–62 (2009)
Acknowledgements
We thank T. Wandless for providing Shld1 compound. This work was supported by a European Research Council Advanced Grant (PlasmoEscape 250320), the French Parasitology consortium ParaFrap (ANR-11-LABX0024), the National Natural Science Foundation of China (NSFC; no. 31271388), the French National Research Agency (13-ISV3-0003-01)–NSFC (no. 81361130411) International Collaboration Project, and the Fundamental Research Funds for the Central Universities of China (20123283). T.N.S. was supported by the Human Frontier Science Program and a European Molecular Biology Organization long-term fellowship. J.C. was supported by the NSFC (no. 81271870). J.G. was supported by the Human Frontier Science Program.
Author information
Authors and Affiliations
Contributions
Q.Z. and A.S. conceived and designed experiments. Q.Z., T.N.S. and R.M.M. performed most of the experiments. Q.Z., T.N.S. and C.H. performed RNA sequencing and data analysis. R.M.M. produced recombinant proteins and performed the exoribonuclease assay in vitro. L.J., X.C., F.W. and H.S. generated constructions, transfectants and parasite material. J.C. and Q.G. collected the field isolates and performed gene transcription analysis. C.S. performed the northern blot assay. L.T. and A.T.R.J. generated the antibodies against individual PfEMP1. J.G. and N.A.M. performed IFA and FACS. Q.Z. and A.S. analysed all the data and wrote the manuscript. All authors discussed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Modelling of crystal structure of conserved catalytic RNase II domains.
a, PfDis3 (putative P. falciparum Dis3, PF3D7_1359300 (PlasmoDB)); b, PfRNase II (PF3D7_0906000 (PlasmoDB)); c, hDis3 (Homo sapiens Dis3, BAF92610.1 (Genbank)); d, yRrp44 (Saccharomyces cerevisiae Dis3, NP_014621.1 (Genbank)). The highly conserved and critical residues in the active centre of the catalytic domain of RNase II are highlighted by pink dots. The amino and carboxy termini are shown in blue and red, respectively.
Extended Data Figure 2 Sequence alignment of RNase II domain of exoribonucleases from P. falciparum, Saccharomyces cerevisiae and Homo sapiens.
The predicted second structures of yRrp44 (top) and PfRNase II (bottom) are shown with arrows (β sheets) and spirals (α helices), respectively. The four critical aspartic residues (D) in the catalytic site that have been shown to be essential for RNase activity are highlighted with red arrows and were replaced by glycine (G) in the PfRNase II-dead-mutant recombinant protein (see Fig. 1d).
Extended Data Figure 3 Biochemical characterization of PfRNase II protein in P. falciparum.
a–c, Schematic representation of the construct of PfRrp4–HA/pLN (a), PfRrp4–HA fusion protein (b), and the recognized site of anti-PfRrp45 antibody (c). d, Western blot of PfRrp4–HA and wild-type 3D7 with anti-HA antibody (left) or anti-PfRrp45 (right). Anti-aldolase antibody was used as a loading control. The full-length proteins are indicated by arrows. e, f, Immunoelectron microscropy assay of PfRNase II in early-stage (e) and mature-stage (f) wild-type 3D7 parasites. Rabbit antibody against PfRNase II was used in this assay (10-nm gold particles). The nuclear membrane is indicated by an arrow. N, nucleus; C, cytoplasm. Scale bar, 500 nm. g, Coomassie staining of three recombinant RNase II domains. h, Sequences of single-stranded (ss) and double-stranded (ds) RNA probes used in exoribonuclease activity analysis in vitro. i, Exoribonuclease activity analysis of recombinant RNase II domains and RNase I (positive control) in vitro with dsRNA probe. j, Exoribonuclease activity analysis of recombinant GST protein (negative control) in vitro with ssRNA probe. Data in d–g, i and j are representative of at least two independent experiments.
Extended Data Figure 4 Strategies of knockout and knockdown approaches of PfRNase II in the 3D7 line.
a, Top: schematic representation of the full-length PfRNase II gene, and the fragments chosen as box 1 or box 2 in pCC1 vector-based knockout constructs. Bottom: various attempts including different combination of box 1 and box 2, parasite lines, and drug on/off selective cyclings are listed in the table. b, Conditional knockout (knockdown) approach of PfRNase II by FKBP destabilization system. c, Three positive clones (G1, H3 and C3), shown in red. d, Western blot assay of PfRNase II protein in the absence of Shld1 in culture. The total extracts of PfRNase II–FKBP-Shld1+ or Shld1− parasites (H3 clone) collected at various time points are indicated on the top of each well. The Shld1 drug was removed in the culture from the ring stage of the first cycle. Rabbit antibody against PfRNase II was used in this assay with anti-aldolase antibody as a loading control. The full-length band of PfRNase II–FKBP is indicated by an arrow. R, ring; S, schizont. Data in c and d are representative of three independent experiments.
Extended Data Figure 5 Comparative analysis of transcriptome changes in PfRNase II–FKBP (H3) and wild-type 3D7-G7 clones by RNA sequencing.
a, Samples and strategies of the analysis. b, Fold change of transcript levels of mitochondrial genes in PfRNase II–FKBP-Shld1+ versus Shld1−. c, List of gene families with a more than fivefold change in transcript levels in the comparison of PfRNase II–FKBP-Shld1+ versus 3D7-G7. Plus sign indicates upregulation of transcript levels. d, Transcription levels of non-coding GC elements and subtelomeric repetitive region (TARE3) in PfRNase II–FKBP-H3 clone with 3D7-G7 as control, determined by qPCR. Samples were harvested at the ring stage. Expression levels were normalized to expression of the seryl-tRNA synthetase gene. Data are represented as means ± s.e.m. for three independent experiments.
Extended Data Figure 6 qPCR analysis of individual var genes in various parasite clones.
a, Transcription profile of 3D7-G7, PfRNase II–FKBP-H3, G1, C3 clones and two subclones of C3 (scB7 and scB1). The predominantly transcribed var genes with distinct ups type are indicated on each column. Expression levels are normalized to expression of the seryl-tRNA synthetase gene. b, Control parasite clones only predominantly transcribe a single var gene. The five subtype var genes and four control genes are shown on the top of the graph. Data are shown as relative copy numbers related to the seryl-tRNA synthetase gene. Samples were harvested at the ring stage.
Extended Data Figure 7 In situ degradation of nascent RNA from promoter regions is linked to the transcriptional regulation of upsA var genes.
a, Switching pattern of var gene family in PfRNase II–FKBP clones during cultivation in vitro for 70 days. The wild-type 3D7-G7 clone was used as control. The predominantly transcribed var genes are indicated beside the columns. Samples were harvested at the ring stage. The five subtype var genes are shown on the top of the graph. Data are shown as relative copy numbers related to the seryl-tRNA synthetase gene. b, Flow cytometry assay of various PfEMP1 expression in mature trophozoite-stage 3D7-G7 and PfRNase II–FKBP-G1 clones. c, Comparative qPCR var transcription analysis from nascent and steady-state mRNA in ring-stage 3D7-G7 wild-type parasites. Primers are designed against different regions of upsA-type var genes. The seryl-tRNA synthetase gene was used as an internal control for the calculation of relative copy numbers. Error bars represent s.e.m. for three independent experiments. d, Transcription analysis of upsA var gene in field isolates from patients with severe malaria or uncomplicated malaria. Data are shown as relative copy numbers related to the seryl-tRNA synthetase gene. **, P < 0.01 (two-tailed Student’s t-test).
Extended Data Figure 8 RNA-seq coverage of var genes in ring-stage wild-type 3D7-G7 clones (green line) and PfRNase II–FKBP H3 clones (red line).
Each plot contains the data of one var locus; that is, from 200 nt upstream of the start codon to 200 nt downstream of the stop codon. Accession numbers are shown above each plot. RNA-seq coverage on sense and antisense strands are shown in the upper and lower panels for each var gene, respectively. The y axis shows the RNA-seq read pileup coverage on a log10 scale, normalized between samples by scaling with the inter-sample mean number of reads mapped to mRNA. The x axis shows the relative position of the var locus (left and right as the 5′ and 3′ ends of the locus, respectively), with the actual genomic range indicated in the axis label in the form [chromosome: strand: start position–end position]. The grey boxes on the x axis refer to the var exons. The repetitiveness of each relative position on the x axis is shown in an extra panel at the bottom, according to the colour scale at the right (from 1 to 20). a–d, Four representative var genes with different ups types and transcription statuses, as indicated on the top of each plot.
Extended Data Figure 9 Chromatin-associated exoribonuclease PfRNase II and plasmodial exosome have distinct functions in P. falciparum.
a, Schematic representation of the PfRNase II–HA fusion protein. b, Examination of the integration event of PfRNase II–HA transfectants by PCR with genomic DNA. c, Western blot of PfRNase II–HA line with anti-HA and anti-aldolase antibodies. d, qPCR analysis of individual var genes in two independent PfRNase II–HA clones. Samples were harvested at the ring stage. The data are shown as relative copy numbers related to the seryl-tRNA synthetase gene. e, ChIP–qPCR of PfRNase II–HA transfectant. The enrichment of distinct var-subtype var genes with anti-HA antibody is shown. Error bars represent s.e.m. for three independent experiments. f, The plasmodial exosome is expected to exert its functions as described in other eukaryotic organisms (i), whereas the additional non-exosome exoribonuclease PfRNase II has evolved as a regulator of expression of upsA-type var genes in P. falciparum (ii). The 3′–5′ exoribonuclease activity may need other helper molecules to access the 3′ end of nascent RNA. Alternatively, a potential N-terminal PIN-like domain of PfRNase II may help to degrade the nascent RNA by its endonuclease activity as described in yeast42. For b and c, data are representative of three independent experiments.
Supplementary information
Supplementary Table 1
This table shows changes of transcripts level in PfRNase II-FKBP H3 clone versus wild-type 3D7-G7 clone. The upregulation and downregulation of 5-fold are shown in two sheets, respectively. (XLS 77 kb)
Supplementary Table 2
This table contains the sequences of the primers used in this study. (XLS 20 kb)
Rights and permissions
About this article
Cite this article
Zhang, Q., Siegel, T., Martins, R. et al. Exonuclease-mediated degradation of nascent RNA silences genes linked to severe malaria. Nature 513, 431–435 (2014). https://doi.org/10.1038/nature13468
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13468
This article is cited by
-
Extracellular vesicles could be a putative posttranscriptional regulatory mechanism that shapes intracellular RNA levels in Plasmodium falciparum
Nature Communications (2023)
-
Erythrocyte miRNA-92a-3p interactions with PfEMP1 as determinants of clinical malaria
Functional & Integrative Genomics (2023)
-
A single point mutation in the Plasmodium falciparum 3′–5′ exonuclease does not alter piperaquine susceptibility
Malaria Journal (2022)
-
Role of chromatin modulation in the establishment of protozoan parasite infection for developing targeted chemotherapeutics
The Nucleus (2021)
-
Actin-related protein Arp4 regulates euchromatic gene expression and development through H2A.Z deposition in blood-stage Plasmodium falciparum
Parasites & Vectors (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.