Abstract
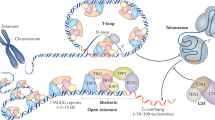
The lengths of human telomeres, which protect chromosome ends from degradation and end fusions1,2, are crucial determinants of cell lifespan3. During embryogenesis and in cancer, the telomerase enzyme counteracts telomeric DNA shortening. As shown in cancer cells, human telomerase binds the shelterin component TPP1 at telomeres4,5 during the S phase of the cell cycle, and adds ∼60 nucleotides in a single round of extension6, after which telomerase is turned off by unknown mechanisms. Here we show that the human CST (CTC1, STN1 and TEN1) complex, previously implicated in telomere protection and DNA metabolism7,8,9,10,11, inhibits telomerase activity through primer sequestration and physical interaction with the protection of telomeres 1 (POT1)–TPP1 telomerase processivity factor12,13. CST competes with POT1–TPP1 for telomeric DNA, and CST–telomeric-DNA binding increases during late S/G2 phase only on telomerase action, coinciding with telomerase shut-off. Depletion of CST allows excessive telomerase activity, promoting telomere elongation. We propose that through binding of the telomerase-extended telomere, CST limits telomerase action at individual telomeres to approximately one binding and extension event per cell cycle. Our findings define the sequence of events that occur to first enable and then terminate telomerase-mediated telomere elongation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jain, D. & Cooper, J. P. Telomeric strategies: means to an end. Annu. Rev. Genet. 44, 243–269 (2010)
de Lange, T. How telomeres solve the end-protection problem. Science 326, 948–952 (2009)
Bodnar, A. G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998)
Abreu, E. et al. TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo. Mol. Cell. Biol. 30, 2971–2982 (2010)
Xin, H. et al. TPP1 is a homologue of ciliate TEBP-β and interacts with POT1 to recruit telomerase. Nature 445, 559–562 (2007)
Zhao, Y. et al. Processive and distributive extension of human telomeres by telomerase under homeostatic and nonequilibrium conditions. Mol. Cell 42, 297–307 (2011)
Miyake, Y. et al. RPA-like mammalian Ctc1-Stn1-Ten1 complex binds to single-stranded DNA and protects telomeres independently of the Pot1 pathway. Mol. Cell 36, 193–206 (2009)
Casteel, D. E. et al. A DNA polymerase-α·primase cofactor with homology to replication protein A-32 regulates DNA replication in mammalian cells. J. Biol. Chem. 284, 5807–5818 (2009)
Surovtseva, Y. V. et al. Conserved telomere maintenance component 1 interacts with STN1 and maintains chromosome ends in higher eukaryotes. Mol. Cell 36, 207–218 (2009)
Nakaoka, H., Nishiyama, A., Saito, M. & Ishikawa, F. Xenopus laevis Ctc1-Stn1-Ten1 (xCST) protein complex is involved in priming DNA synthesis on single-stranded DNA template in Xenopus egg extract. J. Biol. Chem. 287, 619–627 (2012)
Gu, P. et al. CTC1 deletion results in defective telomere replication, leading to catastrophic telomere loss and stem cell exhaustion. EMBO J. 31, 2309–2321 (2012)
Wang, F. et al. The POT1–TPP1 telomere complex is a telomerase processivity factor. Nature 445, 506–510 (2007)
Latrick, C. M. & Cech, T. R. POT1–TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation. EMBO J. 29, 924–933 (2010)
Gao, H., Cervantes, R. B., Mandell, E. K., Otero, J. H. & Lundblad, V. RPA-like proteins mediate yeast telomere function. Nature Struct. Mol. Biol. 14, 208–214 (2007)
Pennock, E., Buckley, K. & Lundblad, V. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell 104, 387–396 (2001)
Qi, H. & Zakian, V. A. The Saccharomyces telomere-binding protein Cdc13p interacts with both the catalytic subunit of DNA polymerase α and the telomerase-associated Est1 protein. Genes Dev. 14, 1777–1788 (2000)
Grossi, S., Puglisi, A., Dmitriev, P. V., Lopes, M. & Shore, D. Pol12, the B subunit of DNA polymerase α, functions in both telomere capping and length regulation. Genes Dev. 18, 992–1006 (2004)
Martin, V., Du, L. L., Rozenzhak, S. & Russell, P. Protection of telomeres by a conserved Stn1-Ten1 complex. Proc. Natl Acad. Sci. US 104, 14038–14043 (2007)
Damm, K. et al. A highly selective telomerase inhibitor limiting human cancer cell proliferation. EMBO J. 20, 6958–6968 (2001)
Cristofari, G. & Lingner, J. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J. 25, 565–574 (2006)
Zhao, Y. et al. Telomere extension occurs at most chromosome ends and is uncoupled from fill-in in human cancer cells. Cell 138, 463–475 (2009)
Cristofari, G. et al. Low- to high-throughput analysis of telomerase modulators with Telospot. Nature Methods 4, 851–853 (2007)
Acknowledgements
We thank P. Reichenbach, K. Ong and S. Feuerhahn for technical help, the PCF-EPFL core facility for protein expression and J. Cooper for discussion. Research in the laboratory of J.L. was supported by the Swiss National Science Foundation, a European Research Council advanced investigator grant (grant agreement no. 232812), the Swiss Cancer League and EPFL.
Author information
Authors and Affiliations
Contributions
L.-Y.C. performed most experiments, S.R. contributed to ChIP assays, L.-Y.C. and J.L. designed the study and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-10. (PDF 3275 kb)
Rights and permissions
About this article
Cite this article
Chen, LY., Redon, S. & Lingner, J. The human CST complex is a terminator of telomerase activity. Nature 488, 540–544 (2012). https://doi.org/10.1038/nature11269
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11269
This article is cited by
-
The regulations of telomerase reverse transcriptase (TERT) in cancer
Cell Death & Disease (2024)
-
A novel mutation of CTC1 leads to telomere shortening in a chinese family with interstitial lung disease
Hereditas (2023)
-
Genetics of human telomere biology disorders
Nature Reviews Genetics (2023)
-
Targeting telomeres: advances in telomere maintenance mechanism-specific cancer therapies
Nature Reviews Cancer (2022)
-
Reconstitution of a telomeric replicon organized by CST
Nature (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.