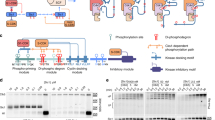
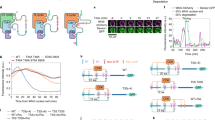
Abstract
Multisite phosphorylation of proteins has been proposed to transform a graded protein kinase signal into an ultrasensitive switch-like response1,2,3,4. Although many multiphosphorylated targets have been identified, the dynamics and sequence of individual phosphorylation events within the multisite phosphorylation process have never been thoroughly studied. In Saccharomyces cerevisiae, the initiation of S phase is thought to be governed by complexes of Cdk1 and Cln cyclins that phosphorylate six or more sites on the Clb5–Cdk1 inhibitor Sic1, directing it to SCF-mediated destruction1,5,6,7,8. The resulting Sic1-free Clb5–Cdk1 complex triggers S phase9. Here, we demonstrate that Sic1 destruction depends on a more complex process in which both Cln2–Cdk1 and Clb5–Cdk1 act in processive multiphosphorylation cascades leading to the phosphorylation of a small number of specific phosphodegrons. The routes of these phosphorylation cascades are shaped by precisely oriented docking interactions mediated by cyclin-specific docking motifs in Sic1 and by Cks1, the phospho-adaptor subunit of Cdk1. Our results indicate that Clb5–Cdk1-dependent phosphorylation generates positive feedback that is required for switch-like Sic1 destruction. Our evidence for a docking network within clusters of phosphorylation sites uncovers a new level of complexity in Cdk1-dependent regulation of cell cycle transitions, and has general implications for the regulation of cellular processes by multisite phosphorylation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nash, P. et al. Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature 414, 514–521 (2001)
Borg, M. et al. Polyelectrostatic interactions of disordered ligands suggest a physical basis for ultrasensitivity. Proc. Natl Acad. Sci. USA 104, 9650–9655 (2007)
Kim, S. Y. & Ferrell, J. E., Jr Substrate competition as a source of ultrasensitivity in the inactivation of Wee1. Cell 128, 1133–1145 (2007)
Thomson, M. & Gunawardena, J. Unlimited multistability in multisite phosphorylation systems. Nature 460, 274–277 (2009)
Tang, X. et al. Suprafacial orientation of the SCFCdc4 dimer accommodates multiple geometries for substrate ubiquitination. Cell 129, 1165–1176 (2007)
Tyers, M. The cyclin-dependent kinase inhibitor p40SIC1 imposes the requirement for Cln G1 cyclin function at Start. Proc. Natl Acad. Sci. USA 93, 7772–7776 (1996)
Verma, R. et al. Phosphorylation of Sic1p by G1 Cdk required for its degradation and entry into S phase. Science 278, 455–460 (1997)
Cross, F. R., Schroeder, L. & Bean, J. M. Phosphorylation of the Sic1 inhibitor of B-type cyclins in Saccharomyces cerevisiae is not essential but contributes to cell cycle robustness. Genetics 176, 1541–1555 (2007)
Schneider, B. L., Yang, Q. H. & Futcher, A. B. Linkage of replication to start by the Cdk inhibitor Sic1. Science 272, 560–562 (1996)
Arvai, A. S., Bourne, Y., Hickey, M. J. & Tainer, J. A. Crystal structure of the human cell cycle protein CksHs1: single domain fold with similarity to kinase N-lobe domain. J. Mol. Biol. 249, 835–842 (1995)
Hadwiger, J. A., Wittenberg, C., Mendenhall, M. D. & Reed, S. I. The Saccharomyces cerevisiae CKS1 gene, a homolog of the Schizosaccharomyces pombe suc1+ gene, encodes a subunit of the Cdc28 protein kinase complex. Mol. Cell. Biol. 9, 2034–2041 (1989)
Reynard, G. J., Reynolds, W., Verma, R. & Deshaies, R. J. Cks1 is required for G1 cyclin-cyclin-dependent kinase activity in budding yeast. Mol. Cell. Biol. 20, 5858–5864 (2000)
Tang, Y. & Reed, S. I. The Cdk-associated protein Cks1 functions both in G1 and G2 in Saccharomyces cerevisiae. Genes Dev. 7, 822–832 (1993)
Patra, D., Wang, S. X., Kumagai, A. & Dunphy, W. G. The Xenopus Suc1/Cks protein promotes the phosphorylation of G2/M regulators. J. Biol. Chem. 274, 36839–36842 (1999)
Deshaies, R. J. & Ferrell, J. E., Jr Multisite phosphorylation and the countdown to S phase. Cell 107, 819–822 (2001)
Kõivomägi, M. et al. Dynamics of Cdk1 substrate specificity during the cell cycle. Mol. Cell 42, 610–623 (2011)
Hao, B., Oehlmann, S., Sowa, M. E., Harper, J. W. & Pavletich, N. P. Structure of a Fbw7-Skp1-cyclin E complex: multisite-phosphorylated substrate recognition by SCF ubiquitin ligases. Mol. Cell 26, 131–143 (2007)
Holt, L. J. et al. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science 325, 1682–1686 (2009)
Kinoshita, E., Yamada, A., Takeda, H., Kinoshita-Kikuta, E. & Koike, T. Novel immobilized zinc(II) affinity chromatography for phosphopeptides and phosphorylated proteins. J. Sep. Sci. 28, 155–162 (2005)
Puig, O. et al. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods 24, 218–229 (2001)
Ubersax, J. A. et al. Targets of the cyclin-dependent kinase Cdk1. Nature 425, 859–864 (2003)
McCusker, D. et al. Cdk1 coordinates cell-surface growth with the cell cycle. Nature Cell Biol. 9, 506–515 (2007)
Mittag, T. et al. Structure/function implications in a dynamic complex of the intrinsically disordered Sic1 with the Cdc4 subunit of an SCF ubiquitin ligase. Structure 18, 494–506 (2010)
Bourne, Y. et al. Crystal structure and mutational analysis of the Saccharomyces cerevisiae cell cycle regulatory protein Cks1: implications for domain swapping, anion binding and protein interactions. Structure 8, 841–850 (2000)
Loog, M. & Morgan, D. O. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature 434, 104–108 (2005)
Acknowledgements
We thank D. Kellogg for strains and L. Peil for advice on mass spectrometry. This work was supported by International Senior Research Fellowship No. 079014/Z/06/Z from the Wellcome Trust (M.Lo.), an installation grant from EMBO and HHMI (M.Lo.), no. 1253, grants no. 6766 from the Estonian Science Foundation (M.Lo.) and SF0180071s07 from Estonian Ministry of Education and Research (M.Lo.), EMP grant no. 08071N from the Norwegian government, and grants from the National Institute of General Medical Sciences (D.O.M.) and National Cancer Institute (S.M.R.).
Author information
Authors and Affiliations
Contributions
M.K., E.V., R.V., A.I., M.Le. and M.Lo. designed and performed the experiments, except for the isothermal calorimetry experiments, performed by E.R.M.B. and S.M.R.; M.Lo. coordinated the project and wrote the manuscript with assistance from D.O.M. and S.M.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Equations, a Supplementary Discussion, Supplementary References, Supplementary Tables 1-4 and Supplementary Figures 1-8 with legends. (PDF 2236 kb)
Rights and permissions
About this article
Cite this article
Kõivomägi, M., Valk, E., Venta, R. et al. Cascades of multisite phosphorylation control Sic1 destruction at the onset of S phase. Nature 480, 128–131 (2011). https://doi.org/10.1038/nature10560
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10560
This article is cited by
-
Regulation of centrosome size by the cell-cycle oscillator in Drosophila embryos
The EMBO Journal (2024)
-
Phosphosite Scanning reveals a complex phosphorylation code underlying CDK-dependent activation of Hcm1
Nature Communications (2023)
-
Positive feedback induces switch between distributive and processive phosphorylation of Hog1
Nature Communications (2023)
-
Controlling protein stability with SULI, a highly sensitive tag for stabilization upon light induction
Nature Communications (2023)
-
An intrinsic temporal order of c-JUN N-terminal phosphorylation regulates its activity by orchestrating co-factor recruitment
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.