Abstract
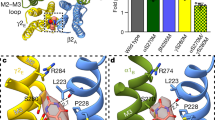
General anaesthetics have enjoyed long and widespread use but their molecular mechanism of action remains poorly understood. There is good evidence that their principal targets are pentameric ligand-gated ion channels1,2 (pLGICs) such as inhibitory GABAA (γ-aminobutyric acid) receptors and excitatory nicotinic acetylcholine receptors, which are respectively potentiated and inhibited by general anaesthetics. The bacterial homologue from Gloeobacter violaceus3 (GLIC), whose X-ray structure was recently solved4,5, is also sensitive to clinical concentrations of general anaesthetics6. Here we describe the crystal structures of the complexes propofol/GLIC and desflurane/GLIC. These reveal a common general-anaesthetic binding site, which pre-exists in the apo-structure in the upper part of the transmembrane domain of each protomer. Both molecules establish van der Waals interactions with the protein; propofol binds at the entrance of the cavity whereas the smaller, more flexible, desflurane binds deeper inside. Mutations of some amino acids lining the binding site profoundly alter the ionic response of GLIC to protons, and affect its general-anaesthetic pharmacology. Molecular dynamics simulations, performed on the wild type (WT) and two GLIC mutants, highlight differences in mobility of propofol in its binding site and help to explain these effects. These data provide a novel structural framework for the design of general anaesthetics and of allosteric modulators of brain pLGICs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Franks, N. P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nature Rev. Neurosci. 9, 370–386 (2008)
Lobo, I. A. & Harris, R. A. Sites of alcohol and volatile anaesthetic action on glycine receptors. Int. Rev. Neurobiol. 65, 53–87 (2005)
Bocquet, N. et al. A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature 445, 116–119 (2007)
Bocquet, N. et al. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457, 111–114 (2009)
Hilf, R. J. C. & Dutzler, R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457, 115–118 (2009)
Weng, Y., Yang, L., Corringer, P. J. & Sonner, J. M. Anaesthetic sensitivity of the Gloeobacter violaceus proton-gated ion channel. Anesth. Analg. 110, 59–63 (2010)
Bhattacharya, A. A., Curry, S. & Franks, N. P. Binding of the general anaesthetics propofol and halothane to human serum albumin. High resolution crystal structures. J. Biol. Chem. 275, 38731–38738 (2000)
Zhang, H., Astrof, N. S., Liu, J., Wang, J. & Shimaoka, M. Crystal structure of isoflurane bound to integrin LFA-1 supports a unified mechanism of volatile anaesthetic action in the immune and central nervous systems. FASEB J. 23, 2735–2740 (2009)
Vedula, L. S. et al. A unitary anaesthetic binding site at high resolution. J. Biol. Chem. 284, 24176–24184 (2009)
Revah, F. et al. Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature 353, 846–849 (1991)
Rubin, M. M. & Changeux, J. P. On the nature of allosteric transitions: implication of non-exclusive ligand binding. J. Mol. Biol. 21, 265–274 (1966)
Hilf, R. J. C. & Dutzler, R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 452, 375–379 (2008)
Haeger, S. et al. An intramembrane aromatic network determines pentameric assembly of Cys-loop receptors. Nature Struct. Mol. Biol. 17, 90–98 (2010)
Villmann, C. et al. Functional complementation of Glra1spd-ot, a glycine receptor subunit mutant, by independently expressed C-terminal domains. J. Neurosci. 29, 2440–2452 (2009)
Nievas, G. A. F., Barrantes, F. J. & Antollini, S. S. Modulation of nicotinic acetylcholine receptor conformational state by free fatty acids and steroids. J. Biol. Chem. 283, 21478–21486 (2008)
da Costa, C. J. B. et al. Anionic lipids allosterically modulate multiple nicotinic acetylcholine receptor conformational equilibria. J. Biol. Chem. 284, 33841–33849 (2009)
Franks, N. P. & Lieb, W. R. Do general anaesthetics act by competitive binding to specific receptors? Nature 310, 599–601 (1984)
Hosie, A. M., Wilkins, M. E., da Silva, H. M. A. & Smart, T. G. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 444, 486–489 (2006)
Violet, J. M., Downie, D. L., Nakisa, R. C., Lieb, W. R. & Franks, N. P. Differential sensitivites of mammalian neuronal and muscle nicotinic acetylcholine receptors to general anesthetics. Anesthesiology 86, 866–874 (1997)
Mihic, S. J. et al. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature 389, 385–389 (1997)
Li, G. et al. Identification of a GABAA receptor anaesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J. Neurosci. 26, 11599–11605 (2006)
Li, G., Chiara, D. C., Cohen, J. B. & Olsen, R. W. Numerous classes of general anaesthetics inhibit etomidate binding to γ-aminobutyric acid type A (GABAA) receptors. J. Biol. Chem. 285, 8615–8620 (2010)
Miyazawa, A., Fujiyoshi, Y. & Unwin, N. Structure and gating mechanism of the acetylcholine receptor pore. Nature 423, 949–955 (2003)
Trudell, J. R. & Bertaccini, E. Comparative modeling of a GABAA α1 receptor using three crystal structures as templates. J. Mol. Graph. Model. 23, 39–49 (2004)
Bali, M., Jansen, M. & Akabas, M. H. GABA-induced intersubunit conformational movement in the GABAA receptor α1M1–β2M3 transmembrane subunit interface: experimental basis for homology modeling of an intravenous anaesthetic binding site. J. Neurosci. 29, 3083–3092 (2009)
Nury, H. et al. One-microsecond molecular dynamics simulation of channel gating in a nicotinic receptor homologue. Proc. Natl Acad. Sci. USA 107, 6275–6280 (2010)
Ziebell, M. R., Nirthanan, S., Husain, S. S., Miller, K. W. & Cohen, J. B. Identification of binding sites in the nicotinic acetylcholine receptor for [3H]azietomidate, a photoactivatable general anaesthetic. J. Biol. Chem. 279, 17640–17649 (2004)
Taly, A., Corringer, P. J., Guedin, D., Lestage, P. & Changeux, J. P. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nature Rev. Drug Discov. 8, 733–750 (2009)
Bertrand, D. & Gopalakrishnan, M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem. Pharmacol. 74, 1155–1163 (2007)
Krause, R. M. et al. Ivermectin: a positive allosteric effector of the α7 neuronal nicotinic acetylcholine receptor. Mol. Pharmacol. 53, 283–294 (1998)
Kabsch, W. Automatic processing of rotation diffraction data from crystals of 21 initially unknown symmetry and cell constants. J. Appl. Cryst. 26, 795–800 (1993)
Collaborative Computational Project The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 (1997)
Bricogne, G. et al. BUSTER, version 2.8.0. Cambridge, UK: Global Phasing. (2009)
Schrödinger, L. L. C. The PyMOL molecular graphics system, version 1.3. 〈http://www.pymol.org〉 (2010)
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996)
Davis, I. W. et al. MolProbity: all-atomcontacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 (2007)
Schuettelkopf, A. W. & van Aalten, D. M. PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. D 60, 1355–1363 (2004)
Dumont, J. N. Oogenesis in Xenopus laevis (Daudin), I. Stages of oocyte development in laboratory maintained animals. J. Morphol. 136, 153–180 (1972)
Barth, L. G. & Barth, L. J. Differentiation of cells of the Rana pipiens gastrula in unconditioned medium. J. Embryol. Exp. Morphol. 7, 210–222 (1959)
Kusano, K., Miledi, R. & Stinnakre, J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J. Physiol. (Lond.) 328, 143–170 (1982)
Krieger, E., Nielsen, J. E., Spronk, C. A. & Vriend, G. Fast empirical pK a prediction by Ewald summation. J. Mol. Graph. Model. 25, 481–486 (2006)
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005)
MacKerell, A. D., Jr et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998)
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993)
Tuckerman, M., Berne, B. J. & Martyna, G. J. Reversible multiple time scale molecular dynamics. J. Chem. Phys. 97, 1990–2001 (1992)
Acknowledgements
This work was supported by the Commission of the European Communities (Neurocypres project; to H.N.), the Louis D. Foundation of the Institut de France, the Network of European Neuroscience Institutes (ENI-NET) and a National Institutes of Health grant NIGMS R01 GM069379 (to J.M.S.). We thank J. Brallet for the gift of desflurane, the European Synchrotron Radiation Facility and Soleil staff for assistance during data collection, and G. Brannigan for providing propofol simulation parameters. Simulations were performed using high-performance computing resources from the Grand Equipement National de Calcul Intensif, Institut du Développement et des Ressources en Informatique Scientifique (GENCI-IDRIS, grant 2009-072292).
Author information
Authors and Affiliations
Contributions
All authors contributed extensively to the work presented in this paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-4 with legends, Supplementary Tables 1-3 and additional references. (PDF 3367 kb)
Rights and permissions
About this article
Cite this article
Nury, H., Van Renterghem, C., Weng, Y. et al. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature 469, 428–431 (2011). https://doi.org/10.1038/nature09647
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09647
This article is cited by
-
High-pressure crystallography shows noble gas intervention into protein-lipid interaction and suggests a model for anaesthetic action
Communications Biology (2022)
-
General anesthesia bullies the gut: a toxic relationship with dysbiosis and cognitive dysfunction
Psychopharmacology (2022)
-
Ligand binding at the protein–lipid interface: strategic considerations for drug design
Nature Reviews Drug Discovery (2021)
-
Comparison of the Conox (qCON) and Sedline (PSI) depth of anaesthesia indices to predict the hypnotic effect during desflurane general anaesthesia with ketamine
Journal of Clinical Monitoring and Computing (2021)
-
Pseudo-Symmetric Assembly of Protodomains as a Common Denominator in the Evolution of Polytopic Helical Membrane Proteins
Journal of Molecular Evolution (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.