Abstract
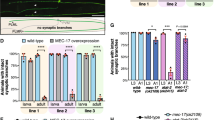
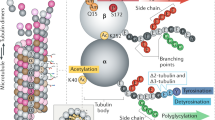
In most eukaryotic cells, subsets of microtubules are adapted for specific functions by post-translational modifications (PTMs) of tubulin subunits. Acetylation of the ε-amino group of K40 on α-tubulin is a conserved PTM on the luminal side of microtubules1 that was discovered in the flagella of Chlamydomonas reinhardtii2,3. Studies on the significance of microtubule acetylation have been limited by the undefined status of the α-tubulin acetyltransferase. Here we show that MEC-17, a protein related to the Gcn5 histone acetyltransferases4 and required for the function of touch receptor neurons in Caenorhabditis elegans5,6, acts as a K40-specific acetyltransferase for α-tubulin. In vitro, MEC-17 exclusively acetylates K40 of α-tubulin. Disruption of the Tetrahymena MEC-17 gene phenocopies the K40R α-tubulin mutation and makes microtubules more labile. Depletion of MEC-17 in zebrafish produces phenotypes consistent with neuromuscular defects. In C. elegans, MEC-17 and its paralogue W06B11.1 are redundantly required for acetylation of MEC-12 α-tubulin, and contribute to the function of touch receptor neurons partly via MEC-12 acetylation and partly via another function, possibly by acetylating another protein. In summary, we identify MEC-17 as an enzyme that acetylates the K40 residue of α-tubulin, the only PTM known to occur on the luminal surface of microtubules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nogales, E., Whittaker, M., Milligan, R. A. & Downing, K. H. High-resolution model of the microtubule. Cell 96, 79–88 (1999)
L'Hernault, S. W. & Rosenbaum, J. L. Chlamydomonas α-tubulin is posttranslationally modified in the flagella during flagellar assembly. J. Cell Biol. 97, 258–263 (1983)
LeDizet, M. & Piperno, G. Identification of an acetylation site of Chlamydomonas α-tubulin. Proc. Natl Acad. Sci. USA 84, 5720–5724 (1987)
Steczkiewicz, K., Kinch, L., Grishin, N. V., Rychlewski, L. & Ginalski, K. Eukaryotic domain of unknown function DUF738 belongs to Gcn5-related N-acetyltransferase superfamily. Cell Cycle 5, 2927–2930 (2006)
Chalfie, M. & Au, M. Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science 243, 1027–1033 (1989)
Zhang, Y. et al. Identification of genes expressed in C. elegans touch receptor neurons. Nature 418, 331–335 (2002)
Verhey, K. J. & Gaertig, J. The tubulin code. Cell Cycle 6, 2152–2160 (2007)
Gaertig, J. et al. Acetylation of lysine 40 in α-tubulin is not essential in Tetrahymena thermophila . J. Cell Biol. 129, 1301–1310 (1995)
Kozminski, K. G., Diener, D. R. & Rosenbaum, J. L. High level expression of nonacetylatable α-tubulin in Chlamydomonas reinhardtii . Cell Motil. Cytoskeleton 25, 158–170 (1993)
Witte, H., Neukirchen, D. & Bradke, F. Microtubule stabilization specifies initial neuronal polarization. J. Cell Biol. 180, 619–632 (2008)
Dompierre, J. P. et al. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J. Neurosci. 27, 3571–3583 (2007)
Creppe, C. et al. Elongator controls the migration and differentiation of cortical neurons through acetylation of α-tubulin. Cell 136, 551–564 (2009)
Nakata, T. & Hirokawa, N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J. Cell Biol. 162, 1045–1055 (2003)
Reed, N. A. et al. Microtubule acetylation promotes kinesin-1 binding and transport. Curr. Biol. 16, 2166–2172 (2006)
Konishi, Y. & Setou, M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nature Neurosci. 12, 559–567 (2009)
Hubbert, C. et al. HDAC6 is a microtubule-associated deacetylase. Nature 417, 455–458 (2002)
North, B. J., Marshall, B. L., Borra, M. T., Denu, J. M. & Verdin, E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol. Cell 11, 437–444 (2003)
Maruta, H., Greer, K. & Rosenbaum, J. L. The acetylation of α-tubulin and its relationship to the assembly and disassembly of microtubules. J. Cell Biol. 103, 571–579 (1986)
Fukushige, T. et al. MEC-12, an α-tubulin required for touch sensitivity in C. elegans . J. Cell Sci. 112, 395–403 (1999)
LeDizet, M. & Piperno, G. Detection of acetylated α-tubulin by specific antibodies. Methods Enzymol. 196, 264–274 (1991)
Barlow, S. B., Gonzalez-Garay, M. L. & Cabral, F. Paclitaxel-dependent mutants have severely reduced microtubule assembly and reduced tubulin synthesis. J. Cell Sci. 115, 3469–3478 (2002)
Bounoutas, A., O'Hagan, R. & Chalfie, M. The multipurpose 15-protofilament microtubules in C. elegans have specific roles in mechanosensation. Curr. Biol. 19, 1362–1367 (2009)
Frøkjaer-Jensen, C. et al. Single-copy insertion of transgenes in Caenorhabditis elegans . Nature Genet. 40, 1375–1383 (2008)
Sun, Z. et al. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131, 4085–4093 (2004)
Wilson, S. W. & Easter, S. S., Jr Stereotyped pathway selection by growth cones of early epiphysial neurons in the embryonic zebrafish. Development 112, 723–746 (1991)
Fox, M. A. & Sanes, J. R. Synaptotagmin I and II are present in distinct subsets of central synapses. J. Comp. Neurol. 503, 280–296 (2007)
Solinger, J. A. et al. The Caenorhabditis elegans Elongator complex regulates neuronal α-tubulin acetylation. PLoS Genet. 6, e1000820 (2010)
Chen, C., Tuck, S. & Bystrom, A. S. Defects in tRNA modification associated with neurological and developmental dysfunctions in Caenorhabditis elegans elongator mutants. PLoS Genet. 5, e1000561 (2009)
Ohkawa, N. et al. N-acetyltransferase ARD1–NAT1 regulates neuronal dendritic development. Genes Cells 13, 1171–1183 (2008)
Shen, Q. et al. NAT10, a nucleolar protein, localizes to the midbody and regulates cytokinesis and acetylation of microtubules. Exp. Cell Res. 315, 1653–1667 (2009)
Mochizuki, K. High efficiency transformation of Tetrahymena using a codon-optimized neomycin resistance gene. Gene 425, 79–83 (2008)
Cassidy-Hanley, D. et al. Germline and somatic transformation of mating Tetrahymena thermophila by particle bombardment. Genetics 146, 135–147 (1997)
Hai, B. & Gorovsky, M. A. Germ-line knockout heterokaryons of an essential α-tubulin gene enable high-frequency gene replacement and a test of gene transfer from somatic to germ-line nuclei in Tetrahymena thermophila . Proc. Natl Acad. Sci. USA 94, 1310–1315 (1997)
Wloga, D. et al. Members of the Nima-related kinase family promote disassembly of cilia by multiple mechanisms. Mol. Biol. Cell 17, 2799–2810 (2006)
Gaertig, J., Gao, Y., Tishgarten, T., Clark, T. G. & Dickerson, H. W. Surface display of a parasite antigen in the ciliate Tetrahymena thermophila . Nature Biotechnol. 17, 462–465 (1999)
Piperno, G. & Fuller, M. T. Monoclonal antibodies specific for an acetylated form of α-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J. Cell Biol. 101, 2085–2094 (1985)
Jerka-Dziadosz, M., Strzyewska-Jowko, I., Wojsa-Lugowska, U., Krawczynska, W. & Krzywicka, A. The dynamics of filamentous structures in the apical band, oral crescent, fission line and the postoral meridional filament in Tetrahymena thermophila revealed by the monoclonal antibody 12G9. Protist 152, 53–67 (2001)
Janke, C. et al. Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science 308, 1758–1762 (2005)
Wloga, D. et al. TTLL3 is a tubulin glycine ligase that regulates the assembly of cilia. Dev. Cell 16, 867–876 (2009)
Brenner, S. The genetics of Caenorhabditis elegans . Genetics 77, 71–94 (1974)
Miller, D. M. & Shakes, D. C. Immunofluorescence microscopy. Methods Cell Biol. 48, 365–394 (1995)
Wloga, D. et al. Glutamylation on α-tubulin is not essential but affects the assembly and functions of a subset of microtubules in Tetrahymena . Eukaryot. Cell 7, 1362–1372 (2008)
Yakovich, A. J., Ragone, F. L., Alfonzo, J. D., Sackett, D. L. & Werbovetz, K. A. Leishmania tarentolae: purification and characterization of tubulin and its suitability for antileishmanial drug screening. Exp. Parasitol. 114, 289–296 (2006)
Kuninger, D., Lundblad, J., Semirale, A. & Rotwein, P. A non-isotopic in vitro assay for histone acetylation. J. Biotechnol. 131, 253–260 (2007)
Acknowledgements
This work was supported by funds from the National Science Foundation (MBC-033965 to J.G.), American Cancer Society (RSG DDC-112979 to S.T.D.), and National Institutes of Health (R01GM074212 to E.T.K., R01AI067981 to N.S.M., R01GM089912 to J.G.). S.T.D. is a Georgia Cancer Coalition Distinguished Investigator. We are grateful to M. Chalfie for the mec-12(e1607) mutant, J. Frankel for 12G10 mAb (available from the Developmental Studies Hybridoma Bank), M. Gorovsky for SG anti-tubulin antibodies, D. Allis for anti-hv1 antibodies, B. Feldman for mismatch morpholinos, and S. T. Dougan laboratory members for advice and assistance with zebrafish experiments.
Author information
Authors and Affiliations
Contributions
J.S.A., D.W., J.K., N.G.S., S.L.-A., S.T.D., E.T.K. and J.G. designed and performed the experiments. N.S.M., S.T.D., E.T.K. and J.G. supervised the work in their respective laboratories. J.G. integrated data and wrote drafts of the paper that were edited by all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Tables 1-2, and Supplementary Figures 1-8, with legends. (PDF 3547 kb)
Supplementary Movie 1
The movie shows zebrafish embryos injected with 1 ng of random sequence morpholinos and recorded at 48 hpf. (MOV 4228 kb)
Supplementary Movie 2
The movie shows zebrafish embryos injected with 1 ng of MEC17-ATG morpholinos and recorded at 48 hpf. (MOV 7074 kb)
Supplementary Movie 3
The movie shows zebrafish embryps injected with 1 ng of MEC17-SP morpholinos and recorded at 48 hpf. (MOV 7043 kb)
Rights and permissions
About this article
Cite this article
Akella, J., Wloga, D., Kim, J. et al. MEC-17 is an α-tubulin acetyltransferase. Nature 467, 218–222 (2010). https://doi.org/10.1038/nature09324
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature09324
This article is cited by
-
The α-tubulin acetyltransferase ATAT1: structure, cellular functions, and its emerging role in human diseases
Cellular and Molecular Life Sciences (2024)
-
Microtubule acetylation dyshomeostasis in Parkinson’s disease
Translational Neurodegeneration (2023)
-
TTLL12 is required for primary ciliary axoneme formation in polarized epithelial cells
EMBO Reports (2023)
-
Nutritional stress-induced regulation of microtubule organization and mRNP transport by HDAC1 controlled α-tubulin acetylation
Communications Biology (2023)
-
Synaptic branch stability is mediated by non-enzymatic functions of MEC-17/αTAT1 and ATAT-2
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.