Abstract
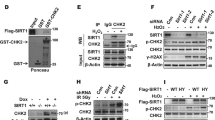
The NAD-dependent protein deacetylase Sir2 (silent information regulator 2) regulates lifespan in several organisms1,2,3. SIRT1, the mammalian orthologue of yeast Sir2, participates in various cellular functions4,5,6,7 and possibly tumorigenesis8. Whereas the cellular functions of SIRT1 have been extensively investigated, less is known about the regulation of SIRT1 activity. Here we show that Deleted in Breast Cancer-1 (DBC1), initially cloned from a region (8p21) homozygously deleted in breast cancers9, forms a stable complex with SIRT1. DBC1 directly interacts with SIRT1 and inhibits SIRT1 activity in vitro and in vivo. Downregulation of DBC1 expression potentiates SIRT1-dependent inhibition of apoptosis induced by genotoxic stress. Our results shed new light on the regulation of SIRT1 and have important implications in understanding the molecular mechanism of ageing and cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lin, S. J., Defossez, P. A. & Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289, 2126–2128 (2000)
Tissenbaum, H. A. & Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410, 227–230 (2001)
Wood, J. G. et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430, 686–689 (2004)
Rodgers, J. T. et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 (2005)
Vaziri, H. et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159 (2001)
Luo, J. et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107, 137–148 (2001)
Brunet, A. et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015 (2004)
Lim, C. S. SIRT1: tumor promoter or tumor suppressor? Med. Hypotheses 67, 341–344 (2006)
Hamaguchi, M. et al. DBC2, a candidate for a tumor suppressor gene involved in breast cancer. Proc. Natl Acad. Sci. USA 99, 13647–13652 (2002)
Guarente, L. & Picard, F. Calorie restriction—the SIR2 connection. Cell 120, 473–482 (2005)
Longo, V. D. & Kennedy, B. K. Sirtuins in aging and age-related disease. Cell 126, 257–268 (2006)
Motta, M. C. et al. Mammalian SIRT1 represses forkhead transcription factors. Cell 116, 551–563 (2004)
Daitoku, H. et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc. Natl Acad. Sci. USA 101, 10042–10047 (2004)
van der Horst, A. et al. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1). J. Biol. Chem. 279, 28873–28879 (2004)
Yeung, F. et al. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380 (2004)
Cohen, H. Y. et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305, 390–392 (2004)
Fulco, M. et al. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol. Cell 12, 51–62 (2003)
Vaquero, A. et al. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol. Cell 16, 93–105 (2004)
Chua, K. F. et al. Mammalian SIRT1 limits replicative life span in response to chronic genotoxic stress. Cell Metab. 2, 67–76 (2005)
Ford, J., Jiang, M. & Milner, J. Cancer-specific functions of SIRT1 enable human epithelial cancer cell growth and survival. Cancer Res. 65, 10457–10463 (2005)
Heltweg, B. et al. Antitumor activity of a small-molecule inhibitor of human silent information regulator 2 enzymes. Cancer Res. 66, 4368–4377 (2006)
Ota, H. et al. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene 25, 176–185 (2006)
Pruitt, K. et al. Inhibition of SIRT1 reactivates silenced cancer genes without loss of promoter DNA hypermethylation. PLoS Genet 2, e40 (2006)
Kuzmichev, A. et al. Composition and histone substrates of polycomb repressive group complexes change during cellular differentiation. Proc. Natl Acad. Sci. USA 102, 1859–1864 (2005)
Chen, W. Y. et al. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell 123, 437–448 (2005)
Sundararajan, R., Chen, G., Mukherjee, C. & White, E. Caspase-dependent processing activates the proapoptotic activity of deleted in breast cancer-1 during tumor necrosis factor-alpha-mediated death signaling. Oncogene 24, 4908–4920 (2005)
Gu, W. & Roeder, R. G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90, 595–606 (1997)
Wang, C. et al. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nature Cell Biol. 8, 1025–1031 (2006)
Nemoto, S., Fergusson, M. M. & Finkel, T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science 306, 2105–2108 (2004)
Araki, T., Sasaki, Y. & Milbrandt, J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305, 1010–1013 (2004)
Acknowledgements
We thank E. White for providing DBC1 expression constructs. We thank T. P. Yao, R. Janknecht, M. Huen and M. H. Sy for providing constructs encoding SIRT1, p300, p53 and Myc, respectively. This work was supported in part by grants from the National Institutes of Health (to J.C). Z.L was supported by a Susan G. Komen Breast Cancer Foundation Research Grant. J.C. is a recipient of an Era of Hope Scholars award from Department of Defense and a member of Mayo Clinic Breast SPORE program.
Author Contributions J.K. and Z.L. performed the experimental work and data analysis. J.K., J.C. and Z.L. wrote the paper. All authors discussed the results and commented on the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
The file contains Supplementary Discussion and Supplementary Figures S1-S5 with Legends. (PDF 8245 kb)
Rights and permissions
About this article
Cite this article
Kim, JE., Chen, J. & Lou, Z. DBC1 is a negative regulator of SIRT1. Nature 451, 583–586 (2008). https://doi.org/10.1038/nature06500
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06500
This article is cited by
-
SIRT1 ISGylation accelerates tumor progression by unleashing SIRT1 from the inactive state to promote its deacetylase activity
Experimental & Molecular Medicine (2024)
-
Multifaceted roles of CCAR family proteins in the DNA damage response and cancer
Experimental & Molecular Medicine (2024)
-
Phospholipase D2 is a positive regulator of sirtuin 1 and modulates p53-mediated apoptosis via sirtuin 1
Experimental & Molecular Medicine (2021)
-
The protein Deleted in Breast Cancer-1 (DBC1) regulates vascular response and formation of aortic dissection during Angiotensin II infusion
Scientific Reports (2020)
-
Acetylation of XPF by TIP60 facilitates XPF-ERCC1 complex assembly and activation
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.