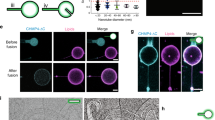
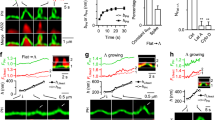
Abstract
Clathrin seems to be dispensable for some endocytic processes and, in several instances, no cytosolic coat protein complexes could be detected at sites of membrane invagination. Hence, new principles must in these cases be invoked to account for the mechanical force driving membrane shape changes. Here we show that the Gb3 (glycolipid)-binding B-subunit of bacterial Shiga toxin induces narrow tubular membrane invaginations in human and mouse cells and model membranes. In cells, tubule occurrence increases on energy depletion and inhibition of dynamin or actin functions. Our data thus demonstrate that active cellular processes are needed for tubule scission rather than tubule formation. We conclude that the B-subunit induces lipid reorganization that favours negative membrane curvature, which drives the formation of inward membrane tubules. Our findings support a model in which the lateral growth of B-subunit–Gb3 microdomains is limited by the invagination process, which itself is regulated by membrane tension. The physical principles underlying this basic cargo-induced membrane uptake may also be relevant to other internalization processes, creating a rationale for conceptualizing the perplexing diversity of endocytic routes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mayor, S. & Pagano, R. E. Pathways of clathrin-independent endocytosis. Nature Rev. Mol. Cell Biol. 8, 603–612 (2007)
Kirkham, M. & Parton, R. G. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim. Biophys. Acta 1745, 273–286 (2005)
Pelkmans, L. & Helenius, A. Endocytosis via caveolae. Traffic 3, 311–320 (2002)
Conner, S. D. & Schmid, S. L. Regulated portals of entry into the cell. Nature 422, 37–44 (2003)
Johannes, L. & Lamaze, C. Clathrin-dependent or not: Is it still the question? Traffic 3, 443–451 (2002)
Nichols, B. J. & Lippincott-Schwartz, J. Endocytosis without clathrin coats. Trends Cell Biol. 11, 406–412 (2001)
Nichols, B. J. et al. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153, 529–541 (2001)
Lauvrak, S. U., Torgersen, M. L. & Sandvig, K. Efficient endosome-to-Golgi transport of Shiga toxin is dependent on dynamin and clathrin. J. Cell Sci. 117, 2321–2331 (2004)
Saint-Pol, A. et al. Clathrin adaptor epsinR is required for retrograde sorting on early endosomal membranes. Dev. Cell 6, 525–538 (2004)
Praefcke, G. J. & McMahon, H. T. The dynamin superfamily: universal membrane tubulation and fission molecules? Nature Rev. Mol. Cell Biol. 5, 133–147 (2004)
Macia, E. et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10, 839–850 (2006)
Sabharanjak, S., Sharma, P., Parton, R. G. & Mayor, S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev. Cell 2, 411–423 (2002)
Kirkham, M. et al. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J. Cell Biol. 168, 465–476 (2005)
Glebov, O. O., Bright, N. A. & Nichols, B. J. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nature Cell Biol. 8, 46–54 (2006)
Roux, A., Uyhazi, K., Frost, A. & De Camilli, P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature 441, 528–531 (2006)
Falguières, T. et al. Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent resistant membranes. Mol. Biol. Cell 12, 2453–2468 (2001)
Bast, D. J., Banerjee, L., Clark, C., Read, R. J. & Brunton, J. L. The identification of three biologically relevant globotriaosyl ceramide receptor binding sites on the Verotoxin 1 B subunit. Mol. Microbiol. 32, 953–960 (1999)
Henley, J. R., Krueger, E. W., Oswald, B. J. & McNiven, M. A. Dynamin-mediated internalization of caveolae. J. Cell Biol. 141, 85–99 (1998)
Farsad, K. & De Camilli, P. Mechanisms of membrane deformation. Curr. Opin. Cell Biol. 15, 372–381 (2003)
McMahon, H. T. & Gallop, J. L. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438, 590–596 (2005)
Zimmerberg, J. & Kozlov, M. M. How proteins produce cellular membrane curvature. Nature Rev. Mol. Cell Biol. 7, 9–19 (2006)
Stein, P. E., Boodhoo, A., Tyrrell, G. J., Brunton, J. L. & Read, R. J. Crystal structure of the cell-binding B oligomer of verotoxin-1 from E. coli. . Nature 355, 748–750 (1992)
Hegnerelle, X. et al. Two-dimensional structures of the Shiga toxin B-subunit and of a chimera bound to the glycolipid receptor Gb3. J. Struct. Biol. 139, 113–121 (2002)
Israelachvili, J. Intramolecular and Surface Forces. 2nd edn, pt 3 (Academic Press, 1991)
Ling, H. et al. Structure of Shiga-like toxin I B-pentamer complexed with an analogue of its receptor Gb3. Biochemistry 37, 1777–1788 (1998)
Sens, P. & Turner, M. S. Theoretical model for the formation of caveolae and similar membrane invaginations. Biophys. J. 86, 2049–2057 (2004)
Fraser, M. E., Chernaia, M. M., Kozlov, Y. V. & James, M. N. Crystal structure of the holotoxin from Shigella dysenteriae at 2.5 Å resolution. Nature Struct. Biol. 1, 59–64 (1994)
Sens, P. & Safran, S. A. Inclusions induced phase separation in mixed lipid film. Eur. Phys. J. E 1, 237–248 (2000)
Jensen, M. H., Morris, E. J. & Simonsen, A. C. Domain shapes, coarsening, and random patterns in ternary membranes. Langmuir 23, 8135–8141 (2007)
Gaus, K. et al. Visualizing lipid structure and raft domains in living cells with two-photon microscopy. Proc. Natl Acad. Sci. USA 100, 15554–15559 (2003)
Gaus, K., Zech, T. & Harder, T. Visualizing membrane microdomains by Laurdan 2-photon microscopy. Mol. Membr. Biol. 23, 41–48 (2006)
Julicher, F. Domain induced budding of vesicles. Phys. Rev. Lett. 70, 2964–2967 (1993)
Baumgart, T., Hess, S. T. & Webb, W. W. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature 425, 821–824 (2003)
Bacia, K., Schwille, P. & Kurzchalia, T. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc. Natl Acad. Sci. USA 102, 3272–3277 (2005)
Antonny, B. Membrane deformation by protein coats. Curr. Opin. Cell Biol. 18, 386–394 (2006)
Reynwar, B. J. et al. Aggregation and vesiculation of membrane proteins by curvature-mediated interactions. Nature 447, 461–464 (2007)
Oda, R., Huc, I., Schmutz, M., Candau, S. J. & MacKintosh, F. C. Tuning bilayer twist using chiral counterions. Nature 399, 566–569 (1999)
Sarasij, R. C. & Rao, M. Tilt texture domains on a membrane and chirality induced budding. Phys. Rev. Lett. 88, 088101 (2002)
Sarasij, R. C., Mayor, S. & Rao, M. Chirality induced budding: a raft-mediated mechanism for endocytosis and morphology of caveolae? Biophys. J. 92, 3140–3158 (2007)
Hancock, J. F. Lipid rafts: contentious only from simplistic standpoints. Nature Rev. Mol. Cell Biol. 7, 456–462 (2006)
Johannes, L., Tenza, D., Antony, C. & Goud, B. Retrograde transport of KDEL-bearing B-fragment of Shiga toxin. J. Biol. Chem. 272, 19554–19561 (1997)
Merrifield, C. J., Perrais, D. & Zenisek, D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121, 593–606 (2005)
Zha, X. et al. Sphingomyelinase treatment induces ATP-independent endocytosis. J. Cell Biol. 140, 39–47 (1998)
Mathivet, L., Cribier, S. & Devaux, P. F. Shape change and physical properties of giant phospholipid vesicles prepared in the presence of an AC electric field. Biophys. J. 70, 1112–1121 (1996)
Menke, M., Gerke, V. & Steinem, C. Phosphatidylserine membrane domain clustering induced by annexin A2/S100A10 heterotetramer. Biochemistry 44, 15296–15303 (2005)
Schmidt, R. R. & Zimmermann, P. Synthesis of D-erythro-sphingosines. Tetrahedr. Lett. 27, 481–484 (1986)
Manzoni, L., Lay, L. & Schmidt, R. R. Synthesis of Lewis A and Lewis X pentasaccharides based on N-trichloroethoxycarbonyl protection. J. Carbohydr. Chem. 17, 739–758 (1998)
Aly, M. R., Rochaix, P., Amessou, M., Johannes, L. & Florent, J. C. Efficient Synthesis of globo- and isoglobotriosides bearing a cinnamoylphenyl tag as novel electrophilic thiol-specific carbohydrate reagents. Carbohydr. Res. 341, 2026–2036 (2006)
Qiu, D. & Schmidt, R. R. Glycosyl imidates. LII. Synthesis of globotriaosylceramide (Gb3) and isoglobotriaosylceramide (isoGb3). Liebigs Ann. Chem. 3, 217–224 (1992)
Figueroa-Perez, S. & Schmidt, R. R. Total synthesis of α-galactosyl cerebroside. Carbohydr. Res. 328, 95–102 (2000)
Acknowledgements
We thank S. Mayor and M. Rao for helpful discussions and sharing unpublished data, M. McNiven for advice, and J.-B. Sibarita and B. Stechmann for assistance with experiments. The following colleagues are acknowledged for providing materials: T. Kirchhausen, M. Bornens, M. McNiven and A. Smith. Our laboratories were supported by: Ligue Nationale contre le Cancer, Association de Recherche Contre le Cancer, Curie Institute (PIC Vectorisation), European Commission (SoftComp), CNRS (ACI Dynamique et réactivité des assemblages biologiques) and the Human Frontier Science Program Organization. W.R. holds a postdoctoral fellowship from the CNRS, and L.B. is supported by a grant from the Direction Générale pour l'Armement (DGA).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-14 with Legends, Legends for Supplementary Movies 1-11 and additional references. (PDF 8199 kb)
Supplementary Movie 1
The file contains Supplementary Movie 1 which shows confocal live cell imaging of tubule growth after injection of 20 nM Alexa488-labeled STxB onto energy-depleted HeLa cells at 37°C. (MOV 1382 kb)
Supplementary Movie 2
The file contains Supplementary Movie 2 which shows confocal live cell imaging of tubule growth at 37°C following binding of 20 nM Alexa488-labeled STxB onto energy-depleted HeLa cells at 4°C. (MOV 1702 kb)
Supplementary Movie 3
The file contains Supplementary Movie 3 which shows confocal live cell imaging of tubule growth at 37°C following binding of 20 nM Alexa488-labeled STxB onto energy-depleted HeLa cells at 4°C. (MOV 3982 kb)
Supplementary Movie 4
The file contains Supplementary Movie 4 which shows evanescent field live cell imaging of tubule scission at 37°C after wash out of energy poisons. (MOV 2105 kb)
Supplementary Movie 5
The file contains Supplementary Movie 5 which shows confocal real time imaging experiment showing tubule formation on a GUV composed of DOPC/cholesterol/Gb3 after injection of Cy3-labeled STxB. (MOV 6177 kb)
Supplementary Movie 6
The file contains Supplementary Movie 6 which shows confocal real time imaging showing tubule dynamics at the equatorial plane of a GUV composed of DOPC/cholesterol/Gb3 at steady state. (MOV 668 kb)
Supplementary Movie 7
The file contains Supplementary Movie 7 which shows spinning-disk confocal real time imaging experiment showing tubule dynamics at steady state in 3D-projection at the equatorial plane of a GUV composed of DOPC/cholesterol/Gb3 during incubation with Cy3-labeled STxB. (MOV 1600 kb)
Supplementary Movie 8
The file contains Supplementary Movie 8 which shows confocal real time imaging experiment at the equatorial plane of a GUV composed of DOPC/cholesterol/Gb3 in the absence of STxB. (MOV 2159 kb)
Supplementary Movie 9
The file contains Supplementary Movie 9 which shows confocal real time imaging experiment on a GUV composed of DOPC/cholesterol/Gb3 onto which the Cy3-labeled W34A mutant was injected. (MOV 3433 kb)
Supplementary Movie 10
The file contains Supplementary Movie 10 which shows confocal real time imaging experiment on a GUV composed of DOPC/cholesterol and Gb3 with a single unsaturated C22:1 acyl chain. (MOV 2371 kb)
Supplementary Movie 11
The file contains Supplementary Movie 11 which shows confocal real time imaging experiment on a GUV composed of DOPC/cholesterol and Gb3 with a saturated C22:0 acyl chain. (MOV 1656 kb)
Rights and permissions
About this article
Cite this article
Römer, W., Berland, L., Chambon, V. et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature 450, 670–675 (2007). https://doi.org/10.1038/nature05996
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05996
This article is cited by
-
Adhesion energy controls lipid binding-mediated endocytosis
Nature Communications (2024)
-
Mesoscale simulation of biomembranes with FreeDTS
Nature Communications (2024)
-
Regulation of membrane protein structure and function by their lipid nano-environment
Nature Reviews Molecular Cell Biology (2023)
-
The bacterial lectin LecA from P. aeruginosa alters membrane organization by dispersing ordered domains
Communications Physics (2023)
-
CLIC and membrane wound repair pathways enable pandemic norovirus entry and infection
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.