Abstract
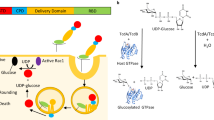
TOXIN A and B, the major virulence factors of Clostridium difficile, are the causative agents of antibiotic-associated pseudomembran-ous colitis. In cultured cell lines their potent cytotoxicity results from their ability to induce disaggregation of the microfilament cytoskeleton1,2. Toxin B acts on the low-molecular-mass GTPase Rho A3,4, which is involved in the regulation of the actin cytoskeleton. We report here that toxin B catalyses the incorporation of up to one mole of glucose per mole of RhoA at the amino acid thre-onine at position 37. The modification was identified and localized by tandem electrospray mass spectrometry. UDP-glucose selectively serves as cosubstrate for the monoglucosylation reaction catalysed by toxin B. Microinjection of RhoA previously glucosyl-ated by toxin B into monolayer cells caused disaggregation of actin filaments, indicating a dominant-negative activity of glucosylated RhoA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lyerly, D. M., Krivan, H. C. & Wilkins, T. D. Clin. Microbiol Rev. 1, 1–18 (1988).
Kelly, C. P., Pothoulakis, C. & LaMont, J. T. New Engl. J. Med. 330, 257–262 (1994).
Just, I. et al. J. biol. Chem. 269, 10706–10712 (1994).
Just, I., Richter, H.-P., Prepens, U., Von Eichel-Streiber, C. & Aktories, K. J. Cell Sci. 107, 1653–1659 (1994).
Wilm, M. & Mann, M. Int. J. Mass Spectrom. Ion Phys. 136, 167–180 (1994).
Bourne, H. R., Sanders, D. A. & McCormick, F. Nature 349, 117–127 (1991).
Pai, E. F. et al. Nature 341, 209–214 (1989).
Pai, E. F. et al. EMBO J. 9, 2351–2359 (1990).
Self, A. J., Paterson, H. F. & Hall, A. Oncogene 8, 655–661 (1993).
Paterson, H. F. et al. Cell Biol. 111, 1001–1007 (1990).
Fenn, J. B., Mann, M., Meng, C. K., Wong, S. F. & Whitehouse, C. M. Science 246, 64–71 (1989).
Roepstorff, P. & Fohlmann, J. J. Biomed. Mass Spectrom. 11, 601 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Just, I., Selzer, J., Wilm, M. et al. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375, 500–503 (1995). https://doi.org/10.1038/375500a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/375500a0
This article is cited by
-
Defined microbial communities and their soluble products protect mice from Clostridioides difficile infection
Communications Biology (2024)
-
AAV-mediated delivery of actoxumab and bezlotoxumab results in serum and mucosal antibody concentrations that provide protection from C. difficile toxin challenge
Gene Therapy (2023)
-
Targeting the Inside of Cells with Biologicals: Toxin Routes in a Therapeutic Context
BioDrugs (2023)
-
Trauma-toxicology: concepts, causes, complications
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
From signal transduction to protein toxins—a narrative review about milestones on the research route of C. difficile toxins
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.