Abstract
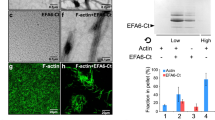
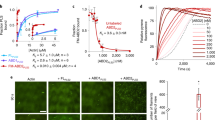
Rho GTPases control actin reorganization and many other cellular functions. Guanine nucleotide-exchange factors (GEFs) activate Rho GTPases by promoting their exchange of GDP for GTP. Trio is a unique Rho GEF, because it has separate GEF domains, GEFD1 and GEFD2, that control the GTPases RhoG/Rac1 and RhoA, respectively. Dbl-homology (DH) domains that are common to GEFs catalyse nucleotide exchange, and pleckstrin-homology (PH) domains localize Rho GEFs near their downstream targets. Here we show that Trio GEFD1 interacts through its PH domain with the actin-filament-crosslinking protein filamin, and localizes with endogenous filamin in HeLa cells. Trio GEFD1 induces actin-based ruffling in filamin-expressing, but not filamin-deficient, cells and in cells transfected with a filamin construct that lacks the Trio-binding domain. In addition, Trio GEFD1 exchange activity is not affected by filamin binding. Our results indicate that filamin, as a molecular target of Trio, may be a scaffold for the spatial organization of Rho-GTPase-mediated signalling pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hall, A. Rho GTPases and the actin cytoskeleton. Science 279, 509–514 (1998).
Nobes, C. D. & Hall, A. Rho, rac and cdc42 GTPases: regulators of actin structures, cell adhesion and motility. Biochem. Soc. Trans. 23, 456–459 (1995).
Gauthier-Rouviere, C. et al. RhoG GTPase controls a pathway that independently activates Rac1 and Cdc42Hs. Mol. Biol. Cell. 9, 1379 –1394 (1998).
Aspenstrom, P. Effectors for the Rho GTPases. Curr. Opin. Cell Biol. 11, 95–102 (1999).
Van Aelst, L. & D'Souza-Schorey, C. Rho GTPases and signaling networks. Genes Dev. 11, 2295 –2322 (1997).
Whitehead, I. P., Campbell, S., Rossman, K. L. & Der, C. J. Dbl family proteins. Biochim. Biophys. Acta 1332, F1–F23 (1997).
Stam, J. C. & Collard, J. G. The DH protein family, exchange factors for Rho-like GTPases. Prog. Mol. Subcell. Biol. 22, 51–83 ( 1999).
Debant, A. et al. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc. Natl Acad. Sci. USA 93, 5466–5471 (1996).
Bellanger, J. M. et al. The two guanine nucleotide exchange factor domains of Trio link the Rac1 and the RhoA pathways in vivo. Oncogene 16, 147–152 (1998).
Blangy, A. et al. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through direct activation of RhoG. J. Cell Sci. 113 , 729–739 (2000).
Liebl, E. C. et al. Dosage-sensitive, reciprocal genetic interactions between the Abl tyrosine kinase and the putative GEF trio reveal trio's role in axon pathfinding. Neuron 26, 107– 118 (2000).
Newsome, T. P. et al. Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell 101, 283–294 (2000).
Awasaki, T. et al. The Drosophila trio plays an essential role in patterning of axons by regulating their directional extension. Neuron 26, 119–131 (2000).
Bateman, J., Shu, H. & Van Vactor, D. The guanine nucleotide exchange factor trio mediates axonal development in the Drosophila embryo. Neuron 26, 93–106 (2000).
Steven, R. et al. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell 92 , 785–795 (1998).
Rohatgi, R. et al. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221–231 (1999).
Kimura, K. et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273, 245– 248 (1996).
Lawler, S. Regulation of actin dynamics: the LIM kinase connection. Curr. Biol. 9, R800–R802 ( 1999).
Chong, L. D., Traynor-Kaplan, A., Bokoch, G. M. & Schwartz, M. A. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell 79, 507 –513 (1994).
Brotschi, E. A., Hartwig, J. H. & Stossel, T. P. The gelation of actin by actin-binding protein. J. Biol. Chem. 253, 8988– 8993 (1978).
Ohta, Y., Suzuki, N., Nakamura, S., Hartwig, J. H. & Stossel, T. P. The small GTPase RalA targets filamin to induce filopodia. Proc. Natl Acad. Sci. USA 96, 2122–2128 ( 1999).
Machesky, L. M. & Gould, K. L. The Arp2/3 complex: a multifunctional actin organizer. Curr. Opin. Cell Biol. 11, 117–121 (1999).
Ohta, Y., Stossel, T. P. & Hartwig, J. H. Ligand-sensitive binding of actin-binding protein to immunoglobulin G Fc receptor I (Fc gamma RI). Cell 67, 275–282 (1991).
Marti, A. et al. Actin-binding protein-280 binds the stress-activated protein kinase (SAPK) activator SEK-1 and is required for tumor necrosis factor-α activation of SAPK in melanoma cells. J. Biol. Chem. 272, 2620–2628 (1997).
Sharma, C. P., Ezzell, R. M. & Arnaout, M. A. Direct interaction of filamin (ABP-280) with the beta 2-integrin subunit CD18. J. Immunol. 154, 3461–3470 (1995).
Gorlin, J. B. et al. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J. Cell Biol. 111 , 1089–1105 (1990).
Sardet, C. et al. E2F-4 and E2F-5, two members of the E2F family, are expressed in the early phases of the cell cycle. Proc. Natl Acad. Sci. USA 92, 2403–2407 ( 1995).
Lebart, M. C., Mejean, C., Roustan, C. & Benyamin, Y. Further characterization of the alpha-actinin-actin interface and comparison with filamin-binding sites on actin. J. Biol. Chem. 268, 5642–5648 (1993).
Cunningham, C. C. et al. Actin-binding protein requirement for cortical stability and efficient locomotion. Science 255, 325– 327 (1992).
Stam, J. C. et al. Targeting of Tiam1 to the plasma membrane requires the cooperative function of the N-terminal pleckstrin homology domain and an adjacent protein interaction domain. J. Biol. Chem. 272, 28447–28454 (1997).
Rebecchi, M. J. & Scarlata, S. Pleckstrin homology domains: a common fold with diverse functions. Annu. Rev. Biophys. Biomol. Struct. 27, 503–528 (1998).
Liu, X. et al. NMR structure and mutagenesis of the N-terminal Dbl homology domain of the nucleotide exchange factor Trio. Cell 95, 269–277 (1998).
Touhara, K., Inglese, J., Pitcher, J. A., Shaw, G. & Lefkowitz, R. J. Binding of G protein beta gamma-subunits to pleckstrin homology domains. J. Biol. Chem. 269, 10217–10220 (1994).
Yao, L. et al. Pleckstrin homology domains interact with filamentous actin. J. Biol. Chem. 274, 19752–19761 (1999).
Umikawa, M. et al. Association of frabin with the actin cytoskeleton is essential for microspike formation through activation of Cdc42 small G protein. J. Biol. Chem. 274, 25197–25200 (1999).
Ott, I., Fischer, E. G., Miyagi, Y., Mueller, B. M. & Ruf, W. A role for tissue factor in cell adhesion and migration mediated by interaction with actin-binding protein 280. J. Cell Biol. 140, 1241– 1253 (1998).
Zhang, W., Han, S. W., McKeel, D. W., Goate, A. & Wu, J. Y. Interaction of presenilins with the filamin family of actin-binding proteins. J. Neurosci. 18, 914–922 (1998).
Loo, D. T., Kanner, S. B. & Aruffo, A. Filamin binds to the cytoplasmic domain of the beta1-integrin. Identification of amino acids responsible for this interaction. J. Biol. Chem. 273, 23304–23312 (1998).
Fox, J. W. et al. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron 21, 1315–1325 (1998).
Letourneau, P. C. & Shattuck, T. A. Distribution and possible interactions of actin-associated proteins and cell adhesion molecules of nerve growth cones. Development 105, 505–519 (1989).
Luo, L., Jan, L. Y. & Jan, Y. N. Rho family GTP-binding proteins in growth cone signalling . Curr. Opin. Neurobiol. 7, 81– 86 (1997).
Hartwig, J. H. et al. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets . Cell 82, 643–653 (1995).
Machesky, L. M. & Hall, A. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J. Cell Biol. 138, 913– 926 (1997).
Vidal, M., Braun, P., Chen, E., Boeke, J. D. & Harlow, E. Genetic characterization of a mammalian protein-protein interaction domain by using a yeast reverse two-hybrid system . Proc. Natl Acad. Sci. USA 93, 10321– 10326 (1996).
Astier, C., Raynaud, F., Lebart, M. C., Roustan, C. & Benyamin, Y. Binding of a native titin fragment to actin is regulated by PIP2. FEBS Lett. 429, 95–98 (1998).
Acknowledgements
We thank S. Schmidt and S. Estrach for discussions, S. Diriong for technical assistance and P. Travo, Head of the CRBM Integrated Imaging Facility, for interest and support. Confocal analysis was carried out at the Centre Regional d'Imagerie Cellulaire, Montpellier, with the help of N. Lautredou. This work was funded by CNRS institutional grants, contracts from the Ligue Nationale contre le Cancer, the Association pour la Recherche contre le Cancer, and USPHS NIH grant HL19429.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bellanger, JM., Astier, C., Sardet, C. et al. The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin. Nat Cell Biol 2, 888–892 (2000). https://doi.org/10.1038/35046533
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35046533
This article is cited by
-
Patterning of the cell cortex by Rho GTPases
Nature Reviews Molecular Cell Biology (2024)
-
Direct and Indirect Effects of Filamin A on Tau Pathology in Neuronal Cells
Molecular Neurobiology (2023)
-
Integrated proteogenomic characterization of urothelial carcinoma of the bladder
Journal of Hematology & Oncology (2022)
-
RhoGEF Trio Regulates Radial Migration of Projection Neurons via Its Distinct Domains
Neuroscience Bulletin (2022)
-
The variants at FLNA and FLNB contribute to the susceptibility of hypertension and stroke with differentially expressed mRNA
The Pharmacogenomics Journal (2021)