Abstract
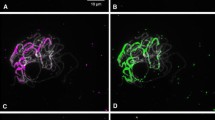
Numerous copies of a 169-base pair DNA sequence (Myzus persicae group repeat; MpR) occur at subtelomeric locations on all chromosomes of three members of the Myzus persicae species group (Myzus persicae, M. antirrhinii, M. certus). MpR occurs in large tandem arrays at both ends of all autosomes of the standard 2n = 12 karyotype, and near one end of the X chromosome (the end opposite to the nucleolar organizer) and is estimated to make up about 5% of the genome (a total of about 200 000 copies). Locations of MpR were compared in various karyotypes to determine the likely nature of the rearrangements (fusions, dissociations, translocations) that are found in this species group which, like other Hemiptera, has holocentric chromosomes that are devoid of morphological markers. Aphid clones heterozygous for autosome dissociations do not have any detectable MpR at 'new' chromosome ends, indicating that this sequence is not involved in 'capping' of chromosomes. However, a clone with a de novo autosome fusion had an interstitial block of MpR marking the point of fusion, and clones heterozygous for an autosomal 1,3 translocation had MpR from autosome 1 translocated to a new site on autosome 3. The isolation from M. antirrhinii of the telomeric repeat TTAGG, which is found in several insect groups, is also reported.
Similar content being viewed by others
References
Bass HW, Marshall WF, Sedat JW, Agard DA, Canda WZ (1997) Telomeres cluster de novo before initiation of synapsis: a three dimensional analysis of telomere positions before and during meiotic prophase. J Cell Biol 137: 5–18.
Bizzaro D, Manicardi GC, Bianchi U (1996) Chromosomal localization of a highly repeated EcoR1 fragment in Megoura viciae (Homoptera, Aphididae) by nick translation and fluorescence in situ hybridization. Chrom Res 4: 392–396.
Blackman RL (1971) Variation in the photoperiodic response within natural populations of Myzus persicae (Sulz.). Bull Entomol Res 60: 533–546.
Blackman RL (1990) The chromosomes of Lachnidae. Acta Phytopath et Ent Hung 25: 273–282.
Blackman RL, Paterson JC (1986) Separation of Myzus (Nectarosiphon) antirrhinii (Macchiati) from Myzus (N.) persicae (Sulzer) and related species in Europe (Homoptera: Aphididae). Syst Entomol 11: 267–276.
Blackman RL, Spence JM (1994) The effects of temperature on aphid morphology, using a multivariate approach. Eur J Entomol 91: 7–22.
Blackman RL, Takada H, Kawakami K (1978) Chromosomal rearrangement involved in the insecticidal resistance of Myzus persicae. Nature 271: 450–452.
Blackman RL, Spence JM, Field LM, Devonshire AL (1995) Chromosomal location of the amplified esterase genes conferring resistance to insecticides in the aphid Myzus persicae. Heredity 75: 297–302.
Blackman RL, Spence JM, Field LM, Javed N, Devine G, Devonshire AL (1996) Inheritance of the amplified esterase genes responsible for insecticide resistance in Myzus persicae (Homoptera: Aphididae). Heredity 77: 154–167.
Collet C, Westerman M (1984) Interspersed distribution patterns of C-bands and satellite DNA in the holocentric chromosomes of Luzula flaccida (Juncaceae). Genetica 63: 175–179.
Dernburg AF, Sedat JW, Hawley RS (1996) Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86: 135–146.
Finston TL, Hebert PD, Foottit RB (1995) Genome size variation in aphids. Insect Biochem Mol Biol 25 :2: 189–196.
Kipling D (1995) The Telomere. Oxford: Oxford University Press.
Lagowski JM, Yu Mei YW, Forrest HS, Laird CD (1973) Dispersity of repeat DNA sequences in Oncopeltus fasciatus, an organism with diffuse centromeres. Chromosoma 43: 349–373.
Lohe AR, Hilliker AJ (1995) Return of the H-word (heterochromatin) [Review]. Curr Opin Genet Dev 5: 746–755.
Loidl J (1990) The initiation of meiotic chromosome pairing: the cytological view. Genome 33: 759–778.
Mason JA, Beissmann H (1995) The unusual telomeres of Drosophila. Trends Genet 11 58–62.
Meyne J, Hirai H, Imai HT (1995) FISH analysis of the telomere sequences of bulldog ants (Myrmecia: Formicidae). Chromosoma 104: 14–18.
Okazaki S, Tsuchida K, Maekawa H, Ishikawa H, Fujiwara H (1993) Identification of a pentanucleotide telomeric sequence (TTAGG)n, in the silkworm Bombyx mori and in other insects. Mol Cell Biol 13: 1424–1432.
Panzera F, Perez R, Panzera Y, Alvarez F, Scvortzoff E, Salvatella R (1995) Karyotype evolution in holocentric chromosomes of three related species of triatomines (Hemiptera: Reduviidae). Chromosom Res 3: 143–150.
Plohl M, Lucijanic-Justic V, Ugarkovic D, Petitpierre E, Juan C (1993) Satellite DNA and heterochromatin of the flour beetle Tribolium confusum. Genome 36: 467–475.
Porter CA (1994) Organization and chromosomal location of repetitive DNA sequences in three species of squamate reptiles. Chromosom Res 2: 263–273.
Rattner JB, Lin Chyi-C (1988) The organization of the centromere and centromeric heterochromatin. In: Verma RS, ed. Heterochromatin: Molecular and Structural Aspects. Cambridge: Cambridge University Press, pp 203–227.
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Scherthan H, Bähler J, Kohli J (1994) Dynamics of chromosome organization and pairing during meiotic prophase in fission yeast. J Cell Biol 127: 273–285.
Scherthan H, Weich S, Schwegler H, Heyting C, Härle M, Cremer T (1996) Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J Cell Biol. 134: 1109–1125.
Schweizer D (1980) Fluorescent chromosome banding in plants: applicatons, mechanisms and implications for chromosome structure. In: Davies DR, Hopwood RA, eds. The Plant Genome, Proc. 4th John Innes Symp. Norwich: John Innes Charity, pp 61–72.
Sunkel CE, Coelho PA (1995) The elusive centromere-sequence divergence and functional conservation. Curr Opin Genet Dev 5: 756–767.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Spence, J.M., Blackman, R.L., Testa, J.M. et al. A 169-base pair tandem repeat DNA marker for subtelomeric heterochromatin and chromosomal rearrangements in aphids of the Myzus persicae group. Chromosome Res 6, 167–175 (1998). https://doi.org/10.1023/A:1009251415941
Issue Date:
DOI: https://doi.org/10.1023/A:1009251415941