Abstract
Introduction
Clinical observations and animal models suggest a critical role for the dynamic regulation of transmural pressure and peristaltic airway smooth muscle contractions for proper lung development. However, it is currently unclear how such mechanical signals are transduced into molecular and transcriptional changes at the cell level. To connect these physical findings to a mechanotransduction mechanism, we identified a known mechanosensor, TRPV4, as a component of this pathway.
Methods
Embryonic mouse lung explants were cultured on membranes and in submersion culture to modulate explant transmural pressure. Time-lapse imaging was used to capture active changes in lung biology, and whole-mount images were used to visualize the organization of the epithelial, smooth muscle, and vascular compartments. TRPV4 activity was modulated by pharmacological agonism and inhibition.
Results
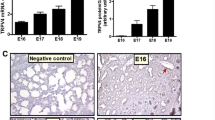
TRPV4 expression is present in the murine lung with strong localization to the epithelium and major pulmonary blood vessels. TRPV4 agonism and inhibition resulted in hyper- and hypoplastic airway branching, smooth muscle differentiation, and lung growth, respectively. Smooth muscle contractions also doubled in frequency with agonism and were reduced by 60% with inhibition demonstrating a functional role consistent with levels of smooth muscle differentiation. Activation of TRPV4 increased the vascular capillary density around the distal airways, and inhibition resulted in a near complete loss of the vasculature.
Conclusions
These studies have identified TRPV4 as a potential mechanosensor involved in transducing mechanical forces on the airways to molecular and transcriptional events that regulate the morphogenesis of the three essential tissue compartments in the lung.






Similar content being viewed by others
Abbreviations
- BPD:
-
Bronchopulmonary dysplasia
- TRPV4:
-
Transient receptor potential cation channel subfamily V member 4
- Pa:
-
Pascal
- PV:
-
Pulmonary vasculature
- PH:
-
Pulmonary hypertension
- HIF1α :
-
Hypoxia-inducible factor 1 alpha
- VEGF:
-
Vascular endothelial growth factor
- ASM:
-
Airway smooth muscle
References
Acarregui, M. J., S. T. Penisten, K. L. Goss, K. Ramirez, and J. M. Snyder. Vascular endothelial growth factor gene expression in human fetal lung in vitro. Am. J. Respir. Cell Mol. Biol. 20:14–23, 1999.
Adapala, R. K., R. J. Thoppil, K. Ghosh, H. C. Cappelli, A. C. Dudley, S. Paruchuri, V. Keshamouni, M. Klagsbrun, J. G. Meszaros, W. M. Chilian, D. E. Ingber, and C. K. Thodeti. Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene 35:314–322, 2016.
Alcorn, D., T. M. Adamson, T. F. Lambert, J. E. Maloney, B. C. Ritchie, and P. M. Robinson. Morphological effects of chronic tracheal ligation and drainage in the fetal lamb lung. J. Anat. 123:649–660, 1977.
Ali, Z., P. Schmidt, J. Dodd, and D. L. Jeppesen. Predictors of bronchopulmonary dysplasia and pulmonary hypertension in newborn children. Dan. Med. J. 60:A4688, 2013.
Alvira, C. M. Aberrant pulmonary vascular growth and remodeling in bronchopulmonary dysplasia. Front. Med. 3:21, 2016.
An, H. S., E. J. Bae, G. B. Kim, B. S. Kwon, J. S. Beak, E. K. Kim, H. S. Kim, J.-H. Choi, C. I. Noh, and Y. S. Yun. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ. J. 40:131–136, 2010.
Benza, R. L., D. P. Miller, R. J. Barst, D. B. Badesch, A. E. Frost, and M. D. McGoon. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 142:448–456, 2012.
Berkelhamer, S. K., K. K. Mestan, and R. H. Steinhorn. Pulmonary hypertension in bronchopulmonary dysplasia. Semin. Perinatol. 37:124–131, 2013.
Berridge, M. J., M. D. Bootman, and H. L. Roderick. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4:517–529, 2003.
Berridge, M. J., P. Lipp, and M. D. Bootman. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1:11–21, 2000.
Bhat, R., A. A. Salas, C. Foster, W. A. Carlo, and N. Ambalavanan. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics 129:e682–e689, 2012.
Brennan, S. C., B. A. Finney, M. Lazarou, A. E. Rosser, C. Scherf, D. Adriaensen, P. J. Kemp, and D. Riccardi. Fetal calcium regulates branching morphogenesis in the developing human and mouse lung: involvement of voltage-gated calcium channels. PLoS ONE 8(11):e80294, 2013.
Chen, C.-K., P.-Y. Hsu, T.-M. Wang, Z.-F. Miao, R.-T. Lin, and S.-H. H. Juo. TRPV4 activation contributes functional recovery from ischemic stroke via angiogenesis and neurogenesis. Mol. Neurobiol. 2017. https://doi.org/10.1007/s12035-017-0625-0.
Compernolle, V., K. Brusselmans, T. Acker, P. Hoet, M. Tjwa, H. Beck, S. Plaisance, Y. Dor, E. Keshet, F. Lupu, B. Nemery, M. Dewerchin, P. Van Veldhoven, K. Plate, L. Moons, D. Collen, and P. Carmeliet. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat. Med. 8:702–710, 2002.
Evans, N. J., and L. N. Archer. Doppler assessment of pulmonary artery pressure during recovery from hyaline membrane disease. Arch. Dis. Child. 66:802–804, 1991.
Featherstone, N. C., M. G. Connell, D. G. Fernig, S. Wray, T. V. Burdyga, P. D. Losty, and E. C. Jesudason. Airway smooth muscle dysfunction precedes teratogenic congenital diaphragmatic hernia and may contribute to hypoplastic lung morphogenesis. Am. J. Respir. Cell Mol. Biol. 35:571–578, 2006.
Featherstone, N. C., E. C. Jesudason, M. G. Connell, D. G. Fernig, S. Wray, P. D. Losty, and T. V. Burdyga. Spontaneous propagating calcium waves underpin airway peristalsis in embryonic rat lung. Am. J. Respir. Cell Mol. Biol. 33:153–160, 2005.
Fewell, J. E., A. A. Hislop, J. A. Kitterman, and P. Johnson. Effect of tracheostomy on lung development in fetal lambs. J. Appl. Physiol. 55:1103–1108, 1983.
Fouron, J. C., J. C. Le Guennec, D. Villemant, G. Perreault, and A. Davignon. Value of echocardiography in assessing the outcome of bronchopulmonary dysplasia of the newborn. Pediatrics 65:529–535, 1980.
Galambos, C., Y.-S. Ng, A. Ali, A. Noguchi, S. Lovejoy, P. A. D’Amore, and D. E. DeMello. Defective pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. Am. J. Respir. Cell Mol. Biol. 27:194–203, 2002.
Galiè, N., H. A. Ghofrani, A. Torbicki, R. J. Barst, L. J. Rubin, D. Badesch, T. Fleming, T. Parpia, G. Burgess, A. Branzi, F. Grimminger, M. Kurzyna, and G. Simonneau. Sildenafil use in pulmonary arterial hypertension (SUPER) study group. Sildenafil citrate therapy for pulmonary arterial hypertension. N. Engl. J. Med. 353:2148–2157, 2005.
Hamanaka, K., M.-Y. Jian, M. I. Townsley, J. A. King, W. Liedtke, D. S. Weber, F. G. Eyal, M. M. Clapp, and J. C. Parker. TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 299:L353–L362, 2010.
Hamanaka, K., M.-Y. Jian, D. S. Weber, D. F. Alvarez, M. I. Townsley, A. B. Al-Mehdi, J. A. King, W. Liedtke, and J. C. Parker. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 293:L923–L932, 2007.
Hara, A., C. J. Chapin, R. Ertsey, and J. A. Kitterman. Changes in fetal lung distension alter expression of vascular endothelial growth factor and its isoforms in developing rat lung. Pediatr. Res. 58:30–37, 2005.
Harding, R., and S. B. Hooper. Regulation of lung expansion and lung growth before birth. J. Appl. Physiol. Bethesda Md 1985(81):209–224, 1996.
Hislop, A. A. Airway and blood vessel interaction during lung development. J. Anat. 201:325–334, 2002.
Hui, A. S., A. L. Bauer, J. B. Striet, P. O. Schnell, and M. F. Czyzyk-Krzeska. Calcium signaling stimulates translation of HIF-alpha during hypoxia. FASEB J. 20:466–475, 2006.
Isakson, B. E., W. H. Evans, and S. Boitano. Intercellular Ca2+ signaling in alveolar epithelial cells through gap junctions and by extracellular ATP. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L221–L228, 2001.
Isakson, B. E., G. J. Seedorf, R. L. Lubman, W. H. Evans, and S. Boitano. Cell-cell communication in heterocellular cultures of alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 29:552–561, 2003.
Jakkula, M., T. D. Le Cras, S. Gebb, K. P. Hirth, R. M. Tuder, N. F. Voelkel, and S. H. Abman. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L600–L607, 2000.
Jesudason, E. C. Small lungs and suspect smooth muscle: congenital diaphragmatic hernia and the smooth muscle hypothesis. J. Pediatr. Surg. 41:431–435, 2006.
Jesudason, E. C. Exploiting mechanical stimuli to rescue growth of the hypoplastic lung. Pediatr. Surg. Int. 23:827–836, 2007.
Jesudason, E. C. Airway smooth muscle: an architect of the lung? Thorax 64:541–545, 2009.
Jesudason, E. C., N. P. Smith, M. G. Connell, D. G. Spiller, M. R. H. White, D. G. Fernig, and P. D. Losty. Developing rat lung has a sided pacemaker region for morphogenesis-related airway peristalsis. Am. J. Respir. Cell Mol. Biol. 32:118–127, 2005.
Jesudason, E. C., N. P. Smith, M. G. Connell, D. G. Spiller, M. R. H. White, D. G. Fernig, and P. D. Losty. Peristalsis of airway smooth muscle is developmentally regulated and uncoupled from hypoplastic lung growth. Am. J. Physiol. Lung Cell. Mol. Physiol. 291:L559–L565, 2006.
Khemani, E., D. B. McElhinney, L. Rhein, O. Andrade, R. V. Lacro, K. C. Thomas, and M. P. Mullen. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 120:1260–1269, 2007.
Kim, H. Y., M.-F. Pang, V. D. Varner, L. Kojima, E. Miller, D. C. Radisky, and C. M. Nelson. Localized smooth muscle differentiation is essential for epithelial bifurcation during branching morphogenesis of the mammalian lung. Dev. Cell 34:719–726, 2015.
Kim, H. Y., V. D. Varner, and C. M. Nelson. Apical constriction initiates new bud formation during monopodial branching of the embryonic chicken lung. Dev. Camb. Engl. 140:3146–3155, 2013.
Lazarus, A., P. M. Del-Moral, O. Ilovich, E. Mishani, D. Warburton, and E. Keshet. A perfusion-independent role of blood vessels in determining branching stereotypy of lung airways. Dev. Camb. Engl. 138:2359–2368, 2011.
Liedtke, W. TRPV channels’ role in osmotransduction and mechanotransduction. Handb. Exp. Pharmacol. 2007. https://doi.org/10.1007/978-3-540-34891-7_28.
Means, A. R. Calcium, calmodulin and cell cycle regulation. FEBS Lett. 347:1–4, 1994.
Miquerol, L., M. Gertsenstein, K. Harpal, J. Rossant, and A. Nagy. Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev. Biol. 212:307–322, 1999.
Moore, K. A., S. Huang, Y. Kong, M. E. Sunday, and D. E. Ingber. Control of embryonic lung branching morphogenesis by the Rho activator, cytotoxic necrotizing factor 1. J. Surg. Res. 104:95–100, 2002.
Moore, K. A., T. Polte, S. Huang, B. Shi, E. Alsberg, M. E. Sunday, and D. E. Ingber. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev. Dyn. 232:268–281, 2005.
Morrisey, E. E., and B. L. M. Hogan. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell 18:8–23, 2010.
Mottet, D., G. Michel, P. Renard, N. Ninane, M. Raes, and C. Michiels. Role of ERK and calcium in the hypoxia-induced activation of HIF-1. J. Cell. Physiol. 194:30–44, 2003.
Mourani, P. M., and S. H. Abman. Pulmonary vascular disease in bronchopulmonary dysplasia: pulmonary hypertension and beyond. Curr. Opin. Pediatr. 25:329–337, 2013.
Muratore, C. S., H. T. Nguyen, M. M. Ziegler, and J. M. Wilson. Stretch-induced upregulation of VEGF gene expression in murine pulmonary culture: a role for angiogenesis in lung development. J. Pediatr. Surg. 35:906–912, 2000; (discussion 912–913).
Nayak, P. S., Y. Wang, T. Najrana, L. M. Priolo, M. Rios, S. K. Shaw, and J. Sanchez-Esteban. Mechanotransduction via TRPV4 regulates inflammation and differentiation in fetal mouse distal lung epithelial cells. Respir. Res. 16:60, 2015.
Nelson, C. M., J. P. Gleghorn, M.-F. Pang, J. M. Jaslove, K. Goodwin, V. D. Varner, E. Miller, D. C. Radisky, and H. A. Stone. Microfluidic chest cavities reveal that transmural pressure controls the rate of lung development. Development 144:4328–4335, 2017.
Olver, R. E., D. V. Walters, and S. M. Wilson. Developmental regulation of lung liquid transport. Annu. Rev. Physiol. 66:77–101, 2004.
Phan, M. N., H. A. Leddy, B. J. Votta, S. Kumar, D. S. Levy, D. B. Lipshutz, S. H. Lee, W. Liedtke, and F. Guilak. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 60:3028–3037, 2009.
Rubin, L. J., D. B. Badesch, R. J. Barst, N. Galie, C. M. Black, A. Keogh, T. Pulido, A. Frost, S. Roux, I. Leconte, M. Landzberg, and G. Simonneau. Bosentan therapy for pulmonary arterial hypertension. N. Engl. J. Med. 346:896–903, 2002.
Schittny, J. C., G. Miserocchi, and M. P. Sparrow. Spontaneous peristaltic airway contractions propel lung liquid through the bronchial tree of intact and fetal lung explants. Am. J. Respir. Cell Mol. Biol. 23:11–18, 2000.
Shinkai, M., T. Shinkai, S. Montedonico, and P. Puri. Effect of VEGF on the branching morphogenesis of normal and nitrofen-induced hypoplastic fetal rat lung explants. J. Pediatr. Surg. 41:781–786, 2006.
Steinhorn, R. H. Neonatal pulmonary hypertension. Pediatr. Crit. Care Med. 11:S79–S84, 2010.
Subhedar, N. V., and N. J. Shaw. Changes in pulmonary arterial pressure in preterm infants with chronic lung disease. Arch. Dis. Child. Fetal Neonatal Ed. 82:F243–F247, 2000.
Taghizadeh, A., and E. O. Reynolds. Pathogenesis of bronchopulmonary dysplasia following hyaline membrane disease. Am. J. Pathol. 82:241–264, 1976.
Tang, N., W. F. Marshall, M. McMahon, R. J. Metzger, and G. R. Martin. Control of mitotic spindle angle by the RAS-regulated ERK1/2 pathway determines lung tube shape. Science 333:342–345, 2011.
Thoppil, R. J., H. C. Cappelli, R. K. Adapala, A. K. Kanugula, S. Paruchuri, and C. K. Thodeti. TRPV4 channels regulate tumor angiogenesis via modulation of Rho/Rho kinase pathway. Oncotarget 7:25849–25861, 2016.
Thorneloe, K. S., A. C. Sulpizio, Z. Lin, D. J. Figueroa, A. K. Clouse, G. P. McCafferty, T. P. Chendrimada, E. S. R. Lashinger, E. Gordon, L. Evans, B. A. Misajet, D. J. Demarini, J. H. Nation, L. N. Casillas, R. W. Marquis, B. J. Votta, S. A. Sheardown, X. Xu, D. P. Brooks, N. J. Laping, and T. D. Westfall. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J. Pharmacol. Exp. Ther. 326:432–442, 2008.
Tomashefski, J. F., H. C. Oppermann, G. F. Vawter, and L. M. Reid. Bronchopulmonary dysplasia: a morphometric study with emphasis on the pulmonary vasculature. Pediatr. Pathol. 2:469–487, 1984.
Unbekandt, M., P.-M. del Moral, F. G. Sala, S. Bellusci, D. Warburton, and V. Fleury. Tracheal occlusion increases the rate of epithelial branching of embryonic mouse lung via the FGF10-FGFR2b-Sprouty2 pathway. Mech. Dev. 125:314–324, 2008.
Walther, F. J., M. J. Benders, and J. O. Leighton. Persistent pulmonary hypertension in premature neonates with severe respiratory distress syndrome. Pediatrics 90:899–904, 1992.
Warburton, D., A. El-Hashash, G. Carraro, C. Tiozzo, F. Sala, O. Rogers, S. De Langhe, P. J. Kemp, D. Riccardi, J. Torday, S. Bellusci, W. Shi, S. R. Lubkin, and E. Jesudason. Lung organogenesis. Curr. Top. Dev. Biol. 90:73–158, 2010.
Yin, J., and W. M. Kuebler. Mechanotransduction by TRP channels: general concepts and specific role in the vasculature. Cell Biochem. Biophys. 56:1–18, 2010.
Acknowledgments
The authors would like to thank Mr. Peter Sariano and Ms. Julia Pelesko for their technical assistance. This work was supported in part by grants from the National Institutes of Health (R01HL133163, R21ES027962, P20GM103446, U54GM104941, S10OD016361), the National Science Foundation (1537256), the Oak Ridge Associated Universities Ralph E. Powe Junior Faculty Enhancement Award (J.P.G.) and the March of Dimes Basil O’Connor Award (5-FY16-33 to J.P.G).
Conflict of interest
Joshua T. Morgan, Wade G. Stewart, Robert A. McKee and Jason P. Gleghorn report no conflicts of interest.
Human and Animal Studies
No human studies were carried out by the authors for this article. All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees at the University of Delaware.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Jason P. Gleghorn is an Assistant Professor at the University of Delaware in the Department of Biomedical Engineering. Gleghorn received his Ph.D. from Cornell University under the mentorship of Lawrence Bonassar. He then completed postdoctoral fellowships at Princeton University with Celeste Nelson and Cornell University with Brian Kirby. During his postdoctoral training, Gleghorn applied microfluidic and microfabrication techniques to identify new physical mechanisms that regulate organ development and he created novel microfluidic systems to isolate rare circulating tumor cells from patient blood samples respectively. His lab, started in 2014 at the University of Delaware, develops and uses microfluidic and microfabrication technologies to determine how cells behave and communicate within multicellular populations to form complex 3D tissues and organs. The long-term goals of this research are to develop techniques to engineer physiologically relevant 3D culture systems with well-defined structure, flows, and cell-cell interactions to study tissue-scale biology and disease. These techniques in combination with what they learn in studies of the native cellular behaviors and interactions in the embryo are used to define new therapeutic approaches for regenerative medicine. Gleghorn’s honors include the ORAU Powe Junior Faculty Award, the March of Dimes Basil O’Connor Award, the UD Bernard Canavan Faculty Research Award, and the BMES CMBE Rising Star Award.
This article is part of the 2018 CMBE Young Innovators special issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12195_2018_538_MOESM1_ESM.tif
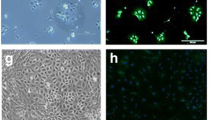
Figure S1: TRPV4 antibody Specificity. Mouse lung epithelial (MLE12) cells were transfected with siRNA specific to TRPV4 or to a non-targeting control (siNT) using Lipofectamine RNAiMAX according to manufacturer instructions. After 72 h, the cells were fixed and stained for TRVP4 and counterstained with DAPI to identify the nuclei. There was a substantial decrease in TRPV4 staining intensity with siTRPV4 compared to siNT treated cells. Scale bars 50 µm. Supplementary material 1 (TIFF 29097 kb)
12195_2018_538_MOESM2_ESM.mp4
Movie S1: TRPV4 regulates active contractility of the developing lung. Embryonic mouse lungs were isolated at E12.5 and cultured on floating membranes for ~ 48 h before 1 Hz imaging. (A) Cultured lungs demonstrated active airway contraction, and this was influenced by TRVP4 modulation. This video spans 100 s, and the control lung visibly contracts at ~ 50 s, whereas 100 nM GSK1016790A (Activator) treated lung visibly contracts at ~ 36 and ~ 68 s, and the lung treat with 10 µM GSK205 (Inhibitor) does not visibly contract. Supplementary material 2 (MP4 1226 kb)
Rights and permissions
About this article
Cite this article
Morgan, J.T., Stewart, W.G., McKee, R.A. et al. The Mechanosensitive Ion Channel TRPV4 is a Regulator of Lung Development and Pulmonary Vasculature Stabilization. Cel. Mol. Bioeng. 11, 309–320 (2018). https://doi.org/10.1007/s12195-018-0538-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-018-0538-7