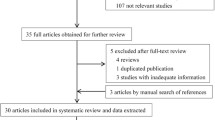
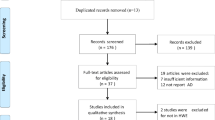
Abstract
Progranulin (PGRN) plays an important role in Alzheimer’s disease (AD) through participating in altering neurite outgrowth and neuronal survival. Previous studies identified that rs5848 in the 3′-untranslated region (3′-UTR) of the PGRN gene (GRN) is strongly associated with AD in Caucasians. In order to assess the involvement of the GRN polymorphism in the risk of late-onset AD (LOAD), we analyzed the genotype and allele distributions of rs5848 in 2350 Han Chinese subjects (AD, 992; control, 1358). The minor T allele of rs5848 was significantly associated with an increased risk of LOAD (P = 0.005, odds ratio (OR) = 1.197, 95 % confidence interval (CI) = 1.057–1.355). Moreover, the association was further validated in the multivariate logistic regression analysis (dominant model: OR = 1.195, P = 0.038, recessive model: OR = 1.386, P = 0.025; additive model: OR = 1.187, P = 0.009). Interestingly, we observed that the interaction between apolipoprotein E (APOE) and rs5848 significantly altered the risk for AD. The rs5848 polymorphism was only significantly associated with LOAD in APOE ε4 allele carriers. Then we included five studies (including the present study) and conducted a meta-analysis which consisted of 3236 cases (male, 1152; female, 2084) and 3405 (male, 1436; female, 1969) controls. The result of the meta-analysis supported T allele of rs5848 within GRN as a risk factor for AD. In conclusion, our results demonstrated that rs5848 polymorphism within GRN was associated with LOAD.




Similar content being viewed by others
References
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362(4):329–344. doi:10.1056/NEJMra0909142
Bettens K, Sleegers K, Van Broeckhoven C (2013) Genetic insights in Alzheimer’s disease. Lancet Neurol 12(1):92–104. doi:10.1016/S1474-4422(12)70259-4
Jiang T, Yu JT, Tian Y, Tan L (2013) Epidemiology and etiology of Alzheimer’s disease: from genetic to non-genetic factors. Curr Alzheimer Res 10(8):852–867
Yu JT, Tan L, Hardy J (2014) Apolipoprotein E in Alzheimer’s disease: an update. Annu Rev Neurosci 37:79–100. doi:10.1146/annurev-neuro-071013-014300
Jiang T, Yu JT, Zhu XC, Zhang QQ, Tan MS, Cao L, Wang HF, Lu J et al (2014) Angiotensin-(1-7) induces cerebral ischaemic tolerance by promoting brain angiogenesis in a Mas/eNOS-dependent pathway. Br J Pharmacol 171(18):4222–4232. doi:10.1111/bph.12770
Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD et al (2011) Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet 43(5):436–441. doi:10.1038/ng.801
Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, Abraham R, Hamshere ML et al (2011) Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet 43(5):429–435. doi:10.1038/ng.803
Tan MS, Jiang T, Tan L, Yu JT (2014) Genome-wide association studies in neurology. Ann Transl Med 2(12):124. doi:10.3978/j.issn.2305-5839.2014.11.12
Tan L, Yu JT, Zhang W, Wu ZC, Zhang Q, Liu QY, Wang W, Wang HF et al (2013) Association of GWAS-linked loci with late-onset Alzheimer’s disease in a northern Han Chinese population. Alzheimers Dement 9(5):546–553. doi:10.1016/j.jalz.2012.08.007
Karch CM, Cruchaga C, Goate AM (2014) Alzheimer’s disease genetics: from the bench to the clinic. Neuron 83(1):11–26. doi:10.1016/j.neuron.2014.05.041
Minami SS, Min SW, Krabbe G, Wang C, Zhou Y, Asgarov R, Li Y, Martens LH et al (2014) Progranulin protects against amyloid beta deposition and toxicity in Alzheimer’s disease mouse models. Nat Med 20(10):1157–1164. doi:10.1038/nm.3672
Chen Y, Li S, Su L, Sheng J, Lv W, Chen G, Xu Z (2015) Association of progranulin polymorphism rs5848 with neurodegenerative diseases: a meta-analysis. J Neurol 262(4):814–822. doi:10.1007/s00415-014-7630-2
Lee MJ, Chen TF, Cheng TW, Chiu MJ (2011) rs5848 variant of progranulin gene is a risk of Alzheimer’s disease in the Taiwanese population. Neurodegener Dis 8(4):216–220. doi:10.1159/000322538
Kamalainen A, Viswanathan J, Natunen T, Helisalmi S, Kauppinen T, Pikkarainen M, Pursiheimo JP, Alafuzoff I et al (2013) GRN variant rs5848 reduces plasma and brain levels of granulin in Alzheimer’s disease patients. J Alzheimers Dis 33(1):23–27. doi:10.3233/jad-2012-120946
Viswanathan J, Makinen P, Helisalmi S, Haapasalo A, Soininen H, Hiltunen M (2009) An association study between granulin gene polymorphisms and Alzheimer’s disease in Finnish population. Am J Med Genet B Neuropsychiatr Genet 150B(5):747–750. doi:10.1002/ajmg.b.30889
Mateo I, Gonzalez-Aramburu I, Pozueta A, Vazquez-Higuera JL, Rodriguez-Rodriguez E, Sanchez-Juan P, Calero M, Dobato JL et al (2013) Reduced serum progranulin level might be associated with Parkinson’s disease risk. Eur J Neurol 20(12):1571–1573. doi:10.1111/ene.12090
Fenoglio C, Galimberti D, Cortini F, Kauwe JS, Cruchaga C, Venturelli E, Villa C, Serpente M et al (2009) Rs5848 variant influences GRN mRNA levels in brain and peripheral mononuclear cells in patients with Alzheimer’s disease. J Alzheimers Dis 18(3):603–612. doi:10.3233/jad-2009-1170
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34(7):939–944
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. doi:10.1007/s10654-010-9491-z
He Z, Bateman A (2003) Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med (Berl) 81(10):600–612. doi:10.1007/s00109-003-0474-3
Petkau TL, Neal SJ, Orban PC, MacDonald JL, Hill AM, Lu G, Feldman HH, Mackenzie IR et al (2010) Progranulin expression in the developing and adult murine brain. J Comp Neurol 518(19):3931–3947. doi:10.1002/cne.22430
Petoukhov E, Fernando S, Mills F, Shivji F, Hunter D, Krieger C, Silverman MA, Bamji SX (2013) Activity-dependent secretion of progranulin from synapses. J Cell Sci 126(Pt 23):5412–5421. doi:10.1242/jcs.132076
Bateman A, Bennett HP (2009) The granulin gene family: from cancer to dementia. Bioessays 31(11):1245–1254. doi:10.1002/bies.200900086
Kortvelyessy P, Gukasjan A, Sweeny-Reed C, Heinze HJ, Thurner L, Bittner DM (2015) Progranulin and amyloid-beta levels: relationship to neuropsychology in frontotemporal and Alzheimer’s disease. J Alzheimers Dis. doi:10.3233/jad-150069
D'Alton S, Lewis J (2014) Understanding the role of progranulin in Alzheimer’s disease. Nat Med 20(10):1099–1100. doi:10.1038/nm.3712
Sleegers K, Brouwers N, Van Broeckhoven C (2010) Role of progranulin as a biomarker for Alzheimer’s disease. Biomark Med 4(1):37–50
Pereson S, Wils H, Kleinberger G, McGowan E, Vandewoestyne M, Van Broeck B, Joris G, Cuijt I et al (2009) Progranulin expression correlates with dense-core amyloid plaque burden in Alzheimer disease mouse models. J Pathol 219(2):173–181. doi:10.1002/path.2580
Antonell A, Gil S, Sanchez-Valle R, Balasa M, Bosch B, Prat MC, Chiollaz AC, Fernandez M et al (2012) Serum progranulin levels in patients with frontotemporal lobar degeneration and Alzheimer’s disease: detection of GRN mutations in a Spanish cohort. J Alzheimers Dis 31(3):581–591. doi:10.3233/jad-2012-112120
Serpente M, Fenoglio C, Clerici F, Bonsi R, Arosio B, Cioffi SM, Rotondo E, Franceschi M et al (2015) Transmembrane protein 106B gene (TMEM106B) variability and influence on progranulin plasma levels in patients with Alzheimer’s disease. J Alzheimers Dis 43(3):757–761. doi:10.3233/jad-141167
Ahmed Z, Mackenzie IR, Hutton ML, Dickson DW (2007) Progranulin in frontotemporal lobar degeneration and neuroinflammation. J Neuroinflammation 4:7. doi:10.1186/1742-2094-4-7
Inestrosa NC, Arenas E (2010) Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci 11(2):77–86. doi:10.1038/nrn2755
Hosokawa M, Arai T, Masuda-Suzukake M, Kondo H, Matsuwaki T, Nishihara M, Hasegawa M, Akiyama H (2015) Progranulin reduction is associated with increased tau phosphorylation in P301L tau transgenic mice. J Neuropathol Exp Neurol 74(2):158–165. doi:10.1097/NEN.0000000000000158
Karch CM, Jeng AT, Skorupa T, Cruchaga C, Goate AM (2013) Novel progranulin variants do not disrupt progranulin secretion and cleavage. Neurobiol Aging 34(11):2538–2540. doi:10.1016/j.neurobiolaging.2013.05.004
Rademakers R, Eriksen JL, Baker M, Robinson T, Ahmed Z, Lincoln SJ, Finch N, Rutherford NJ et al (2008) Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet 17(23):3631–3642. doi:10.1093/hmg/ddn257
Hsiung GY, Fok A, Feldman HH, Rademakers R, Mackenzie IR (2011) rs5848 polymorphism and serum progranulin level. J Neurol Sci 300(1–2):28–32. doi:10.1016/j.jns.2010.10.009
Dickson DW, Baker M, Rademakers R (2010) Common variant in GRN is a genetic risk factor for hippocampal sclerosis in the elderly. Neurodegener Dis 7(1–3):170–174. doi:10.1159/000289231
Brouwers N, Sleegers K, Engelborghs S, Maurer-Stroh S, Gijselinck I, van der Zee J, Pickut BA, Van den Broeck M et al (2008) Genetic variability in progranulin contributes to risk for clinically diagnosed Alzheimer disease. Neurology 71(9):656–664. doi:10.1212/01.wnl.0000319688.89790.7a
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81471309, 81371406, 81571245, and 81501103), the Shandong Provincial Outstanding Medical Academic Professional Program, Qingdao Key Health Discipline Development Fund, Qingdao Outstanding Health Professional Development Fund, and Shandong Provincial Collaborative Innovation Center for Neurodegenerative Disorders.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Written informed consent was obtained from each participant or their guardian, and our study was approved by the Ethical Committee of Qingdao Municipal Hospital (2009-05-06-003).
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Hui-Min Xu and Lin Tan should be regarded as co-first authors.
Rights and permissions
About this article
Cite this article
Xu, HM., Tan, L., Wan, Y. et al. PGRN Is Associated with Late-Onset Alzheimer’s Disease: a Case–Control Replication Study and Meta-analysis. Mol Neurobiol 54, 1187–1195 (2017). https://doi.org/10.1007/s12035-016-9698-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9698-4