Abstract
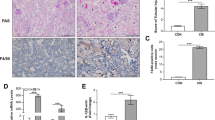
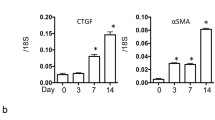
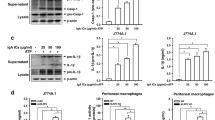
To produce proinflammatory master cytokine IL-1β in macrophages, two stimulation pathways are needed including TLRs-NF-κB axis and NLRPs/ASC-caspase-1 axis. Different signals including exogenous and endogenous trigger inflammatory response distinctly. Among them, the role of endogenous stimulators of inflammation is poorly understood. As a component of hemoglobin, free heme is released when hemolysis or extensive cell damage occur which results in inflammatory response. Here, we find that heme induces IL-1β secretion through activating NLRP3 inflammasome in macrophages. Heme activates NLRP3 through P2X receptors, especially the P2X7R and P2X4R. Most importantly, significantly enhancement of heme level and activation of NLRPs/ASC-caspase-1 axis were observed in mice kidney after unilateral ureteral obstruction which could be inhibited by enforced expression of heme oxygenase-1 (HO-1). Our study proves that heme is a potential danger activator of NLRP3 inflammasome that plays an essential role in IL-1β secretion during kidney inflammation and provides new insight into the mechanism of innate immune initiation. Further investigation will be beneficial to develop new molecular target and molecular diagnosis indicator in therapy of kidney inflammation.




Similar content being viewed by others
References
Collins, A. J., et al. (2009). The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol, 4(Suppl 1), S5–S11.
Anders, H. J., & Muruve, D. A. (2011). The inflammasomes in kidney disease. J Am Soc Nephrol, 22(6), 1007–1018.
Rock, K. L., et al. (2010). The sterile inflammatory response. Ann Rev Immunol, 28, 321–342.
Takeuchi, O., & Akira, S. (2010). Pattern recognition receptors and inflammation. Cell, 140(6), 805–820.
Sanz, A. B., et al. (2010). NF-kappaB in renal inflammation. J Am Soc Nephrol, 21(8), 1254–1262.
Babelova, A., et al. (2009). Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem, 284(36), 24035–24048.
Ting, J. P., et al. (2008). The NLR gene family: a standard nomenclature. Immunity, 28(3), 285–287.
Schroder, K., & Tschopp, J. (2010). The inflammasomes. Cell, 140(6), 821–832.
Martinon, F., Mayor, A., & Tschopp, J. (2009). The inflammasomes: guardians of the body. Annu Rev Immunol, 27, 229–265.
Joshi, V. D., et al. (2002). Role of caspase 1 in murine antibacterial host defenses and lethal endotoxemia. Infect Immun, 70(12), 6896–6903.
Wang, C., et al. (2012). Quercetin and allopurinol ameliorate kidney injury in STZ-treated rats with regulation of renal NLRP3 inflammasome activation and lipid accumulation. PLoS ONE, 7(6), e38285.
Tsai, P. Y., et al. (2011). Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic Biol Med, 51(3), 744–754.
Hu, Q. H., et al. (2012). Allopurinol, quercetin and rutin ameliorate renal NLRP3 inflammasome activation and lipid accumulation in fructose-fed rats. Biochem Pharmacol, 84(1), 113–125.
Muller-Eberhard, U., et al. (1968). Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood, 32(5), 811–815.
Jacob, H. S. (1994). Newly recognized causes of atherosclerosis: the role of microorganisms and of vascular iron overload. J Lab Clin Med, 123(6), 808–816.
Pamplona, A., et al. (2007). Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat Med, 13(6), 703–710.
Graca-Souza, A. V., et al. (2002). Neutrophil activation by heme: implications for inflammatory processes. Blood, 99(11), 4160–4165.
Wagener, F. A., et al. (2003). Different faces of the heme–heme oxygenase system in inflammation. Pharmacol Rev, 55(3), 551–571.
White, L. R., et al. (2007). The characterization of alpha5-integrin expression on tubular epithelium during renal injury. Am J Physiol Renal Physiol, 292(2), F567–F576.
Schaefer, L., et al. (2005). The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest, 115(8), 2223–2233.
Vilaysane, A., et al. (2010). The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol, 21(10), 1732–1744.
Soares, M. P., et al. (2004). Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol, 172(6), 3553–3563.
Seixas, E., et al. (2009). Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proc Natl Acad Sci USA, 106(37), 15837–15842.
Martinon, F., & Tschopp, J. (2007). Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death and Differ, 14(1), 10–22.
Martinon, F., et al. (2007). NALP inflammasomes: a central role in innate immunity. Semin Immunopathol, 29(3), 213–229.
Mariathasan, S., et al. (2004). Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature, 430(6996), 213–218.
Darisipudi, M. N., et al. (2012). Uromodulin triggers IL-1beta-dependent innate immunity via the NLRP3 inflammasome. J Am Soc Nephrol, 23(11), 1783–1789.
Mariathasan, S., et al. (2006). Cryopyrin activates the inflammasome in response to toxins and ATP. Nature, 440(7081), 228–232.
Solini, A., et al. (2013). The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation. J Pathol, 231(3), 342–353.
Guo, C., et al. (2007). Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol, 72(6), 1447–1456.
Tenhunen, R., Marver, H. S., & Schmid, R. (1968). The enzymatic conversion of hemetobilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA, 61, 748.
Matzinger, P. (1994). Tolerance, danger, and the extended family. Ann Rev Immunol, 12, 991–1045.
Beutler, B. (2004). Inferences, questions and possibilities in Toll-like receptor signalling. Nature, 430(6996), 257–263.
Hornung, V., et al. (2008). Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol, 9(8), 847–856.
Darisipudi, M. N., et al. (2011). Polyene macrolide antifungal drugs trigger interleukin-1beta secretion by activating the NLRP3 inflammasome. PLoS ONE, 6(5), e19588.
Allam, R., et al. (2011). Cutting edge: cyclic polypeptide and aminoglycoside antibiotics trigger IL-1beta secretion by activating the NLRP3 inflammasome. J Immunol, 186(5), 2714–2718.
Mulay, S. R., et al. (2013). Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1beta secretion. J Clin Invest, 123(1), 236–246.
Lorenz, G., Darisipudi, M. N., & Anders, H.J. (2013). Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol Dial Transplant.
Figueiredo, R. T., et al. (2007). Characterization of heme as activator of Toll-like receptor 4. Journal of Biological Chemistry, 282(28), 20221–20229.
Jeney, V., et al. (2002). Pro-oxidant and cytotoxic effects of circulating heme. Blood, 100(3), 879–887.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Qianwei Li and Weihua Fu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, Q., Fu, W., Yao, J. et al. Heme Induces IL-1β Secretion Through Activating NLRP3 in Kidney Inflammation. Cell Biochem Biophys 69, 495–502 (2014). https://doi.org/10.1007/s12013-014-9823-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-9823-9