Abstract
Type 1 diabetes (T1D) is a chronic autoimmune disease that leads to progressive destruction of pancreatic beta cells. Compared to healthy controls, a characteristic feature of patients with T1D is the presence of self-reactive T cells with a memory phenotype. These autoreactive memory T cells in both the CD4+ and CD8+ compartments are likely to be long-lived, strongly responsive to antigenic stimulation with less dependence on costimulation for activation and clonal expansion, and comparatively resistant to suppression by regulatory T cells (Tregs) or downregulation by immune-modulating agents. Persistence of autoreactive memory T cells likely contributes to the difficulty in preventing disease progression in new-onset T1D and maintaining allogeneic islet transplants by regular immunosuppressive regimens. The majority of immune interventions that have demonstrated some success in preserving beta cell function in the new-onset period have been shown to deplete or modulate memory T cells. Based on these and other considerations, preservation of residual beta cells early after diagnosis or restoration of beta cell mass by use of stem cell or transplantation technology will require a successful strategy to control the autoreactive memory T cell compartment, which could include depletion, inhibition of homeostatic cytokines, induction of hyporesponsiveness, or a combination of these approaches.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–300.
Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371:1972–82. An important study showing that even with good glycemic control, T1D results in substantial excess mortality.
Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–6.
Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32:457–67.
Wang P, Fiaschi-Taesch NM, Vasavada RC, et al. Diabetes mellitus—advances and challenges in human β-cell proliferation. Nat Rev Endocrinol. 2015;11:201–12.
Coppieters KT, Wiberg A, Tracy AM, et al. Immunology in the clinic review series: focus on type 1 diabetes and viruses: the role of viruses in type 1 diabetes: a difficult dilemma. Clin Exp Immunol. 2012;168:5–11.
Schneider A, Rieck M, Sanda S, et al. The effector T cells of diabetic subjects aqre resistant to regulation via CD4+FOXP3+ regulatory T cells. J Immunol. 2008;181:7350–5.
Danke NA, Yang J, Greenbaum C, et al. Comparative study of GAD65-specific CD4+ T cells in healthy and type 1 diabetic subjects. J Autoimmun. 2005;25:303–11.
Monti P, Scirpoli M, Rigamonti A, et al. Evidence for in vivo primed and expanded autoreactive T cells as a specific feature of patients with type 1 diabetes. J Immunol. 2007;179:5785–92.
Oling V, Reijonen H, Simell O, et al. Autoantigen-specific memory CD4+ T cells are prevalent early in progression to type 1 diabetes. Cell Immunol. 2012;273:133–9. One of several studies showing that islet-reactive CD4 + T cells are found in the peripheral blood of healthy subjects but that these cells tend to have a naïve phenotype versus the memory phenotype found in subjects with T1D autoimmunity.
Skowera A, Ladell K, McLaren JE, et al. β-cell-specific CD8 T cell phenotype in type 1 diabetes reflects chronic autoantigen exposure. Diabetes. 2015;64:916–25. An important study showing that beta cell-specific CD8 + T cells in patients with T1D tend to have a stem memory phenotype and are characterized by a highly skewed oligoclonal T cell receptor repertoire.
Sutherland DE, Sibley R, Xu XZ, et al. Twin-to-twin pancreas transplantation: reversal and reenactment of the pathogenesis of type I diabetes. Trans Assoc Am Physicians. 1984;97:80–7.
Sibley RK, Sutherland DER, Goetz F, et al. Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest. 1985;53:132–44.
Sutherland DER, Goetz FC, Sibley RK. Recurrence of diabetes in pancreas transplants. Diabetes. 1989;38 Suppl 1:85–7.
Tyden G, Reinholt FP, Sundkvist G, et al. Recurrence of autoimmune diabetes mellitus in recipients of cadaveric pancreatic grafts. New Engl J Med. 1996;335:860–2.
Roep BO, Stobbe I, Duinkerken G, et al. Auto- and alloimmune reactivity to human islet allografts trasnplanted into type 1 diabetic patients. Diabetes. 1999;48:484–90.
Pinkse GGM, Tysma OHM, Bergen CAM, et al. Autoreactive CD8 T cells associated with β cell destruction in type 1 diabetes. Proc Natl Acad Sci U S A. 2005;102:18425–30.
Laughlin E, Burke G, Pugliese A, et al. Recurrence of autoreactive antigen-specific CD4+ T cells in autoimmune diabetes after pancreas transplantation. Clin Immunol. 2008;128:23–30.
Monti P, Scirpoli M, Maffi P, et al. Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells. J Clin Invest. 2008;118:1806–14.
Vendrame F, Pileggi A, Laughlin E, et al. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes. 2010;59:947–57.
Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63.
Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nat Immunol. 2014;15:1104–15. An excellent recent review of our understanding of memory T cell differentiation and function.
Devarajan P, Chen Z. Autoimmune effector memory T cells: the bad and the good. Immunol Res. 2013;57:12–22. A useful review of the memory T cell compartment in autoimmunity, with an emphasis on Tem cells.
Chee J, Ko HJ, Skowera A, et al. Effector-memory T cells develop in islets and report islet pathology in type 1 diabetes. J Immunol. 2014;192:572–80. An important study in the NOD model demonstrating that acquisition of the effector-memory phenotype by islet-specific CD8 + T cells occurs in infiltrated islets, followed by emigration to peripheral lymphoid tissue.
Orban T, Beam CA, Xu P, et al. Reduction in CD4 central memory T-cell subset in costimulation modulator abatacept-treated patients with recent-onset type 1 diabetes is associated with slower C-peptide decline. Diabetes. 2014;63:3449–57. The first demonstration that CD4 + Tcm cells are down-modulated by costimulation blockade (abatacept) and that this correlates with C-peptide preservation.
Surh CD, Sprent J. Homeostasis of naïve and memory T cells. Immunity. 2008;29:848–62.
Calzascia T, Pellegrini M, Lin A, et al. CD4 T cells, lymphopenia, and IL-7 in a multistep pathway to autoimmunity. Proc Natl Acad Sci U S A. 2008;105:2999–3004.
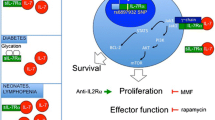
Penaranda C, Kuswanto W, Hofmann J, et al. IL-7 receptor blockade reverses autoimmune diabetes by promoting inhibition of effector/memory T cells. Proc Natl Acad Sci U S A. 2012;109:12668–73. Together with the study by Lee et al. (ref. 29), the first demonstration that IL-7 receptor blockade robustly reverses diabetes in NOD mice by inhibiting Tem cells and upregulating PD-1 expression.
Lee LF, Logriono K, Tu GH, et al. Anti-IL-7 receptor-α reverses established type 1 diabetes in nonobese diabetic mice by modulating effector T-cell function. Proc Natl Acad Sci U S A. 2012;109:12674–9. See comment relating to ref. 28.
Murakami N, Riella LV. Co-inhibitory pathways and their importance in immune regulation. Transplantation. 2014;98:3–14. An excellent recent review of co-inhibitory and co-stimulatory pathways in immune regulation.
Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–42.
Pauken KE, Jenkins MK, Azuma M, Fife BT. PD-1, but not PD-L1, expressed by islet-reactive CD4+ T cells suppresses infiltration of the pancreas during type 1 diabetes. Diabetes. 2013;62:2859–69. One of several studies indicating that the PD-1 pathway may be important in maintaining peripheral tolerance in the NOD model of diabetes autoimmunity.
Ansari MJ, Salama AD, Chitnis T, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63–9.
Fife BT, Guleria I, Gubbels Bupp M, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–47.
Li R, Lee J, Kim MS, et al. PD-L1-driven tolerance protects neurogenin3-induced islet neogenesis to reverse established type 1 diabetes in NOD mice. Diabetes. 2015;64:529–40.
Waldmann TA. Targeting the interleukin-15 system in rheumatoid arthritis. Arthritis Rheum. 2005;52:2585–8.
Baslund B, Tvede N, Danneskiold-Samsoe B, et al. Targeting interleukin-15 in patients with rheumatoid arthritis. A proof-of-concept study. Arthritis Rheum. 2005;52:2686–92.
Finch DK, Midha A, Buchanan CL, et al. Identification of a potent anti-IL-15 antibody with opposing mechanisms of action in vitro and in vivo. Br J Pharmacol. 2011;162:480–90.
Krueger GG. Selective targeting of T cell subsets: focus on alefacept—a remittive therapy for psoriasis. Expert Opin Biol Ther. 2002;2:431–41.
Chamian F, Lin SL, Lee E, et al. Alefacept (anti-CD2) causes a selective reduction in circulating effector memory T cells (Tem) and relative preservation of central memory T cells (Tcm) in psoriasis. J Transl Med. 2007;5:27.
Haider AS, Lowes MA, Gardner H, et al. Novel insight into the agonistic mechanism of alefacept in vivo: differentially expressed genes may serve as biomarkers of response in psoriasis patients. J Immunol. 2007;178:7442–9.
Punch JD, Lin J, Bluestone J, et al. CD2 and CD3 receptor-mediated tolerance: constraints on T cell activation. Transplantation. 1999;67:741–8.
Chamian F, Lowes MA, Lin SL, et al. Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris. Proc Natl Acad Sci U S A. 2005;102:2075–80.
Ellis CN, Krueger GG. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med. 2001;345:248–55.
Rigby MR, DiMeglio LA, Rendell MS, et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol. 2013;1:284–94. The first demonstration in the clinic that targeting the CD2 pathway leads to robust depletion of Tem cells, an increase in the Treg/Tem ratio, and preservation of C-peptide in new-onset T1D.
Barlow AK, Like AA. Anti-CD2 monoclonal antibodies prevent spontaneous and adoptive transfer of diabetes in the BB/Wor rat. Am J Pathol. 1992;141:1043–51.
Bai Y, Fu S, Honig S, et al. CD2 is a dominant target for allogeneic responses. Am J Transplant. 2002;2:618–26.
Chavin KD, Qin L, Lin J, et al. Combined anti-CD2 and anti-CD3 receptor monoclonal antibodies induce donor-specific tolerance in a cardiac transplant model. J Immunol. 1993;151:7249–59.
Chavin KD, Qin L, Lin J, et al. Combination anti-CD2 and anti-CD3 monoclonal antibodies induce tolerance while altering interleukin-2, interleukin-4, tumor necrosis factor, and transforming growth factor-beta production. Ann Surg. 1993;218:492–501.
Chavin KD, Qin L, Lin J, et al. Anti-CD2 and anti-CD3 monoclonal antibodies synergize to prolong allograft survival with decreased side effects. Transplantation. 1993;55:901–8.
Chavin KD, Qin L, Lin J, et al. Anti-CD2 monoclonal antibodies synergize with anti-CD3 to prolong allograft survival and decrease cytokine production. Transplant Proc. 1993;25:823–4.
Kapur S, Khanna A, Sharma VK, et al. CD2 antigen targeting reduces intragraft expression of mRNA-encoding granzyme B and IL-10 and induces tolerance. Transplantation. 1996;62:249–55.
Kapur S, Sharma V, Khanna A, et al. Regulation of the anti-allograft response by targeting the CD2 antigen: a potential strategy for the creation of transplant tolerance. Surg Technol Int. 1996;5:233–40.
Krueger GG, Callis KP. Development and use of alefacept to treat psoriasis. J Am Acad Dermatol. 2003;49:S87–97.
Miller GT, Hochman PS, Meier W, et al. Specific interaction of lymphocyte function-associated antigen 3 with CD2 can inhibit T cell responses. J Exp Med. 1993;178:211–22.
Kuhns MS, Davis MM, Garcia KC. Deconstructing the form and function of the TCR/CD3 complex. Immunity. 2006;24:133–9.
Chatenoud L, Thervet E, Primo J, et al. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A. 1994;91:123–7.
Daifotis AG, Koenig S, Chatenoud L, et al. Anti-CD3 clinical trials in type 1 diabetes mellitus. Clin Immunol. 2013;149:268–78.
Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–8.
Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–608.
Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–9.
Keymeulen B, Walter M, Mathieu C, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53:614–23.
Sherry N, Hagopian W, Ludvigsson J, et al. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487–97.
Ambery P, Donner TW, Biswas N, et al. Efficacy and safety of low-dose otelixizumab anti-CD3 monoclonal antibody in preserving C-peptide secretion in adolescent type 1 diabetes: DEFEND-2, a randomized, placebo-controlled, double-blind, multi-centre study. Diabet Med. 2014;31:399–402.
Aronson R, Gottlieb PA, Christiansen JS, et al. Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase III study in recent-onset human type 1 diabetes. Diabetes Care. 2014;37:2746–54.
Herold KC, Gitelman SE, Ehlers MR, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766–74. The first trial in new-onset T1D to clearly show that response to anti-CD3 therapy is heterogenous and that a responder group can be identified.
Penaranda C, Tang Q, Bluestone JA. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J Immunol. 2011;187:2015–22.
Herold KC, Gitelman SE, Willi SM, et al. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia. 2013;56:391–400. The first demonstration that CD8 + Tcm cells are modulated in patients with recent-onset T1D who are responders to anti-CD3 therapy.
Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–52.
Linsley PS, Brady W, Urnes M, et al. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–9.
Ford ML, Adams AB, Pearson TC. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nat Rev Nephrol. 2014;10:14–24.
Webster RM. The immune checkpoint inhibitors: where are we now? Nat Rev Drug Discov. 2014;13:883–4.
Linsley PS, Wallace PM, Johnson J, et al. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–5.
Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–82.
Orban T, Bundy B, Becker DJ, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–9.
Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13.
Eisenbarth GS, Srikanta S, Jackson R, et al. Anti-thymocyte globulin and prednisone immunotherapy of recent onset type 1 diabetes mellitus. Diabetes Res. 1985;2:271–6.
Gitelman SE, Gottlieb PA, Rigby MR, et al. Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2013;1:306–16. The first adequately powered trial in new-onset T1D showing that treatment with antithymocyte globulin monotherapy does not deplete Tem cells and fails to preserve C-peptide.
Long SA, Rieck M, Sanda S, et al. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs beta-cell function. Diabetes. 2012;61:2340–8.
Mordes JP, Bortell R, Doukas J, et al. The BB/Wor rat and the balance hypothesis of autoimmunity. Diabetes Metab Rev. 1996;12:103–9.
Smilek DE, Ehlers MR, Nepom GT. Restoring the balance: immunotherapeutic combinations for autoimmune disease. Dis Model Mech. 2014;7:503–13.
Haller MJ, Gitelman SE, Gottlieb PA, et al. Anti-thymocyte globulin/G-CSF treatment preserves β cell function in patients with established type 1 diabetes. J Clin Invest. 2015;125:448–55. An important pilot study demonstrating that combination therapy with low-dose antithymocyte globulin plus G-CSF in new-onset T1D appears to preserve C-peptide, possibly by inducing a favorable Treg/Teff ratio.
Gregori S, Mangia P, Bacchetta R, et al. An anti-CD45RO/RB monoclonal antibody modulates T cell responses via induction of apoptosis and generation of regulatory T cells. J Exp Med. 2005;201:1293–305.
Rus H, Pardo CA, Hu L, et al. The voltage-gated potassium channel Kv1.3 is highly expressed on inflammatory infiltrates in multiple sclerosis brain. Proc Natl Acad Sci U S A. 2005;102:11094–9.
Beeton C, Wulff H, Standifer NE, et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci U S A. 2006;103:17414–9.
Kineta Announces Promising Top-line Clinical Results for Dalazatide; Target Implicated in Broad Array of Autoimmune Diseases. http://www.kinetabio.com/press_releases/PressRelease20150505_dalazatide.pdf: Kineta, Inc.; 2015.
Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12:191–200.
Acknowledgments
This publication was supported in part by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Number UM1AI109565. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Mario Ehlers declares that he has no conflict of interest. Mark Rigby is an employee of Janssen R&D, Pharmaceutical Companies of Johnson & Johnson.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Treatment of Type 1 Diabetes
Rights and permissions
About this article
Cite this article
Ehlers, M.R., Rigby, M.R. Targeting Memory T Cells in Type 1 Diabetes. Curr Diab Rep 15, 84 (2015). https://doi.org/10.1007/s11892-015-0659-5
Published:
DOI: https://doi.org/10.1007/s11892-015-0659-5