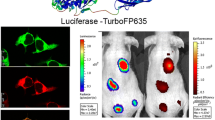
Abstract
Purpose
Our goal is to develop a simple, quantitative, robust method to compare the efficacy of imaging reporter genes in culture and in vivo. We describe an adenoviral vector–liver transduction procedure and compare the luciferase reporter efficacies.
Procedures
Alternative reporter genes are expressed in a common adenoviral vector. Vector amounts used in vivo are based on cell culture titrations, ensuring that the same transduction efficacy is used for each vector. After imaging, in vivo and in vitro values are normalized to hepatic vector transduction using quantitative real-time PCR.
Results
We assayed standard firefly luciferase (FLuc), enhanced firefly luciferase (EFLuc), luciferase 2 (Luc2), humanized Renilla luciferase (hRLuc), Renilla luciferase 8.6-535 (RLuc8.6), and a membrane-bound Gaussia luciferase variant (extGLuc) in cell culture and in vivo. We observed greater than 100-fold increase in bioluminescent signal for both EFLuc and Luc2 when compared to FLuc and greater than 106-fold increase for RLuc8.6 when compared to hRLuc. ExtGLuc was not detectable in liver.
Conclusions
Our findings contrast, in some cases, with conclusions drawn in prior comparisons of these reporter genes and demonstrate the need for a standardized method to evaluate alternative reporter genes in vivo. Our procedure can be adapted for reporter genes that utilize alternative imaging modalities (fluorescence, bioluminescence, MRI, SPECT, PET).





Similar content being viewed by others
References
Ishikawa TO, Jain NK, Taketo MM, Herschman HR (2006) Imaging cyclooxygenase-2 (Cox-2) gene expression in living animals with a luciferase knock-in reporter gene. Mol Imaging Biol 8:171–187
Xie X, Xia W, Li Z et al (2007) Targeted expression of BikDD eradicates pancreatic tumors in noninvasive imaging models. Cancer Cell 12:52–65
Contag CH, Jenkins D, Contag PR, Negrin RS (2000) Use of reporter genes for optical measurements of neoplastic disease in vivo. Neoplasia 2:41–52
Contag CH, Contag PR, Mullins JI, Spilman SD, Stevenson DK, Benaron DA (1995) Photonic detection of bacterial pathogens in living hosts. Mol Microbiol 18:593–603
Rettig GR, Mcanuff M, Liu D, Kim JS, Rice KG (2006) Quantitative bioluminescence imaging of transgene expression in vivo. Anal Biochem 355:90–94
Jenkins DE, Oei Y, Hornig YS et al (2003) Bioluminescent imaging (BLI) to improve and refine traditional murine models of tumor growth and metastasis. Clin Exp Metastasis 20:733–744
Vooijs M, Jonkers J, Lyons S, Berns A (2002) Noninvasive imaging of spontaneous retinoblastoma pathway-dependent tumors in mice. Cancer Res 62:1862–1867
Edinger M, Cao YA, Verneris MR, Bachmann MH, Contag CH, Negrin RS (2003) Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood 101:640–648
Dothager RS, Flentie K, Moss B, Pan MH, Kesarwala A, Piwnica-Worms D (2009) Advances in bioluminescence imaging of live animal models. Curr Opin Biotechnol 20:45–53
Sjolinder H, Jonsson AB (2007) Imaging of disease dynamics during meningococcal sepsis. PLoS One 2:e241
Hwang S, Wu TT, Tong LM et al (2008) Persistent gammaherpesvirus replication and dynamic interaction with the host in vivo. J Virol 82:12498–12509
Saeji JP, Arrizabalaga G, Boothroyd JC (2008) A cluster of four surface antigens specifically expressed in bradyzoites, SAG2CDXY, plays an important role in Toxyoplasma gondii. Infect Immun 76:2402–2410
Li Z, Wu JC, Sheikh AY et al (2007) Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation 116:I46–I54
Van Der Bogt KE, Sheikh AY, Schrepfer S et al (2008) Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation 118:S121–S129
Feichtinger GA, Morton TJ, Zimmermann A et al (2011) Enhanced reporter gene assay for the detection of osteogenic differentiation. Tissue Eng Part C Methods 17:401–410
Binkowski B, Fan F, Wood K (2009) Engineered luciferases for molecular sensing in living cells. Curr Opin Biotechnol 20:14–18
Lorenz WW, Mccann RO, Longiaru M, Cormier MJ (1991) Isolation and expression of a cDNA encoding Renilla reniformis luciferase. Proc Natl Acad Sci USA 88:4438–4442
Ugarova NN (1989) Luciferase of Luciola mingrelica fireflies. Kinetics and regulation mechanism. J Biolumin Chemilumin 4:406–418
Rehemtulla A, Stegman LD, Cardozo SJ et al (2000) Rapid and quantitative assessment of cancer treatment response using in vivo bioluminescence imaging. Neoplasia 2:491–495
Sherf BA, Wood KV (1994) Firefly luciferase engineered for improved genetic reporting. Promega Notes 49:14–21
Rabinovich BA, Ye Y, Etto T et al (2008) Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proc Natl Acad Sci USA 105:14342–14346
Loening AM, Wu AM, Gambhir SS (2007) Red-shifted Renilla reniformis luciferase variants for imaging in living subjects. Nat Methods 4:641–643
Tannous BA, Kim DE, Fernandez JL, Weissleder R, Breakefield XO (2005) Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol Ther 11:435–443
Zhao H, Doyle TC, Coquoz O, Kalish F, Rice BW, Contag CH (2005) Emission spectra of bioluminescent reporters and interaction with mammalian tissue determine the sensitivity of detection in vivo. J Biomed Opt 10:41210
Rice BW, Cable MD, Nelson MB (2001) In vivo imaging of light-emitting probes. J Biomed Opt 6:432–440
Santos EB, Yeh R, Lee J et al (2009) Sensitive in vivo imaging of T cells using a membrane-bound Gaussia princeps luciferase. Nat Med 15:338–344
Kimura T, Hiraoka K, Kasahara N, Logg CR (2010) Optimization of enzyme-substrate pairing for bioluminescence imaging of gene transfer using Renilla and Gaussia luciferases. J Gene Med 12:528–537
Morgan JR (2002) Gene therapy protocols. Humana, Totowa
Maizel JV Jr, White DO, Scharff MD (1968) The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology 36:115–125
Gil JS, Gallaher SD, Berk AJ (2010) Delivery of an EBV episome by a self-circularizing helper-dependent adenovirus: long-term transgene expression in immunocompetent mice. Gene Ther 17:1288–1293
Gallaher SD, Gil JS, Dorigo O, Berk AJ (2009) Robust in vivo transduction of a genetically stable Epstein–Barr virus episome to hepatocytes in mice by a hybrid viral vector. J Virol 83:3249–3257
Kontermann RE, Korn T, Jerome V (2003) Recombinant adenoviruses for in vivo expression of antibody fragments. Methods Mol Biol 207:421–433
Sohlenius-Sternbeck AK (2006) Determination of the hepatocellularity number for human, dog, rabbit, rat and mouse livers from protein concentration measurements. Toxicol In Vitro 20:1582–1586
Bhaumik S, Gambhir SS (2002) Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc Natl Acad Sci USA 99:377–382
Zhuang Y, Butler B, Hawkins E et al (2001) New synthetic Renilla gene and assay system increase expression, reliability and sensitivity. Promega Notes 79:6–11
Weissleder R (2001) A clearer vision for in vivo imaging. Nat Biotechnol 19:316–317
Matthews JC, Hori K, Cormier MJ (1977) Purification and properties of Renilla reniformis luciferase. Biochemistry 16:85–91
Loening AM, Fenn TD, Wu AM, Gambhir SS (2006) Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng Des Sel 19:391–400
Acknowledgements
We thank Arthur Catapang for technical help, David Stout and Waldemar Ladno for advice and assistance with the optical imaging experiments, and Arion Chattziioannou and the members of the Herschman lab for helpful discussions. This study was funded by the National Cancer Institute In Vivo Cellular and Molecular Imaging Center (ICMIC) award P50 CA086306 (HRH). JG is supported by a Scholars in Oncologic Medical Imaging (SOMI) fellowship from the National Cancer Institute (Award R25T CA098010).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gil, J.S., Machado, H.B. & Herschman, H.R. A Method to Rapidly and Accurately Compare the Relative Efficacies of Non-invasive Imaging Reporter Genes in a Mouse Model and its Application to Luciferase Reporters. Mol Imaging Biol 14, 462–471 (2012). https://doi.org/10.1007/s11307-011-0515-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-011-0515-1