Abstract
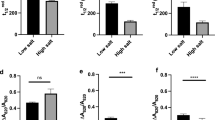
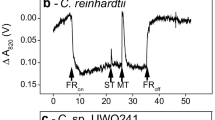
Under environmental stress, plants and algae employ a variety of strategies to protect the photosynthetic apparatus and maintain photostasis. To date, most studies on stress acclimation have focused on model organisms which possess limited to no tolerance to stressful extremes. We studied the ability of the Antarctic alga Chlamydomonas sp. UWO 241 (UWO 241) to acclimate to low temperature, high salinity or high light. UWO 241 maintained robust growth and photosynthetic activity at levels of temperature (2 °C) and salinity (700 mM NaCl) which were nonpermissive for a mesophilic sister species, Chlamydomonas raudensis SAG 49.72 (SAG 49.72). Acclimation in the mesophile involved classic mechanisms, including downregulation of light harvesting and shifts in excitation energy between photosystem I and II. In contrast, UWO 241 exhibited high rates of PSI-driven cyclic electron flow (CEF) and a larger capacity for nonphotochemical quenching (NPQ). Furthermore, UWO 241 exhibited constitutively high activity of two key ascorbate cycle enzymes, ascorbate peroxidase and glutathione reductase and maintained a large ascorbate pool. These results matched the ability of the psychrophile to maintain low ROS under short-term photoinhibition conditions. We conclude that tight control over photostasis and ROS levels are essential for photosynthetic life to flourish in a native habitat of permanent photooxidative stress. We propose to rename this organism Chlamydomonas priscuii.









Similar content being viewed by others
Availability of data and material
The data supporting the findings of this study are available from the corresponding author (RMK) upon request.
Code availability
Not applicable.
Abbreviations
- A820 :
-
Absorbance at 820 nm
- ΦPSII:
-
Yield of photosystem II
- APX:
-
Ascorbate peroxidase
- AsA-GSH:
-
Ascorbate–glutathione pathway
- C:
-
Control
- CAT:
-
Catalase
- CBB:
-
Calvin Benson Bassham
- CEF:
-
Cyclic electron flow
- FR:
-
Far red
- F V/F M :
-
Maximum photosynthetic efficiency of photosystem II
- GR:
-
Glutathione reductase
- H2DCFDA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- HL:
-
High light
- HS:
-
High salt
- LHCI:
-
Light harvesting complex I
- LHCII:
-
Light harvesting complex II
- LHCSR:
-
Light harvesting complex stress related protein
- LT:
-
Low temperature
- qL:
-
Photochemical quenching
- MDHAR:
-
Dehydroascorbate reductase
- NBT:
-
Nitroblue tetrazolium
- NPQ:
-
Nonphotochemical quenching
- PGR5:
-
Proton gradient regulation 5 protein
- PGRL1:
-
PGR5-like protein 1
- pmf:
-
Proton motive force
- PQ:
-
Plastoquinone
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- ROS:
-
Reactive oxygen species
- RT-qPCR:
-
Real time quantitative PCR
- SOD:
-
Super oxide dismutase
- t ½ red :
-
Half-time for P700 re-reduction
References
Alboresi A, Gerotto C, Giacometti GM, Bassi R, Morosinotto T (2010) Physcomitrella patens mutants affected on heat dissipation clarify the evolution of photoprotection mechanisms upon land colonization. Proc Natl Acad Sci 107:11128–11133
Aldesuquy HS, Baka ZA, El-Shehaby O, Ghanem HE (2013) Growth, lipid peroxidation and antioxidant enzyme activities as a selection criterion for the salt tolerance of wheat cultivars irrigated by seawater. Phyton 53:153–165
Apel K, Hirt H (2004) Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Asada K (1996) In: Baker NR (ed) Photosynthesis and the Environment. Springer, Berlin, p 123
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photon. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Asada K (2000) The water-water cycle as alternative photon and electron sinks. Philos Trans R Soc Lond B Biol Sci 355(1402):1419–1431
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141(2):391–396
Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24(1):23–58
Bartoli CG, Buet A, Grozeff GG, Galatro A, Simontacchi M (2017) Ascorbate-glutathione cycle and abiotic stress tolerance in plants. In: Hossain MA (ed) Ascorbic acid in plant growth, development and stress tolerance. Springer, Berlin, pp 177–200
Borowitzka MA (2018) The ‘stress’ concept in microalgal biology—homeostasis, acclimation and adaptation. J Appl Phycol 30:2815–2825
Bulte L, Gans P, Febeille F, Wollman F (1990) ATP control on state transitions in vivo in Chlamydomonas reinhardtii. Biochem Biophys Acta 1020:72–80
Chaux F, Peltier G, Johnson X (2015) A security network in PSI photoprotection: regulation of photosynthetic control, NPQ and O2 photoreduction by cyclic electron flow. Front Plant Sci 6:875
Chen Q, Zhang M, Shen S (2011) Effect of salt on malondialdehyde and antioxidant enzymes in seedling roots of Jerusalem artichoke (Helianthus tuberosus L.). Acta Physiol Plant 33(2):273–278
Cook G, Teufel A, Kalra I, Li W, Wang X, Priscu J, Morgan-Kiss R (2019) The antarctic psychrophiles Chlamydomonas spp. UWO241 and ICE-MDV exhibit differential restructuring of photosystem I in response to iron. Photosynth Res 141:209–228
Cournac L, Latouche G, Cerovic Z, Redding K, Ravenel J, Peltier G (2002) In vivo interactions between photosynthesis, mitorespiration, and chlororespiration in Chlamydomonas reinhardtii. Plant Physiol 129(4):1921–1928
Cvetkovska M, Hüner NP, Smith DR (2017) Chilling out: the evolution and diversification of psychrophilic algae with a focus on Chlamydomonadales. Polar Biol 40:1169–1184
Cvetkovska M, Orgnero S, Hüner NP, Smith DR (2019) The enigmatic loss of light-independent chlorophyll biosynthesis from an Antarctic green alga in a light-limited environment. New Phytol 222(2):651–656
DalCorso G, Pesaresi P, Masiero S, Aseeva E, Schünemann D, Finazzi G, Joliot P, Barbato R, Leister D (2008) A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 132:273–285
Ensminger I, Busch F, Hüner NPA (2006) Photostasis and cold acclimation: sensing low temperature through photosynthesis. Physiol Plant 126(1):28–44. https://doi.org/10.1111/j.1399-3054.2006.00627.x
Falk S, Krol M, Maxwell DP, Rezansoff DA, Gray GR, Hüner NPA (1994) Changes in in vivo fluorescence quenching in rye and barley as a function of reduced PSII light harvesting antenna size. Physiol Plant 91:551–558
Falk S, Maxwell D, Gray G, Rezansoff D, Hüner N (1993) Photosynthetic acclimation to low temperature in higher plants and algae. Curr Top Bot Res 1:281–292
Förster B, Osmond CB, Pogson BJ (2005) Improved survival of very high light and oxidative stress is conferred by spontaneous gain-of-function mutations in Chlamydomonas. Biochim Et Biophys Acta-Bioenerg 1709(1):45–57
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133(1):21–25
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide-and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant 100(2):241–254
Foyer CH, Noctor G (2012) Managing the cellular redox hub in photosynthetic organisms. Plant Cell Environ 35(2):199–201
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155(1):93–100
Galindo V, Gosselin M, Lavaud J, Mundy CJ, Else B, Ehn J, Babin M, Rysgaard S (2017) Pigment composition and photoprotection of Arctic sea ice algae during spring. Mar Ecol Prog Ser 585:49–69
Gest N, Gautier H, Stevens R (2013) Ascorbate as seen through plant evolution: the rise of a successful molecule? J Exp Bot 64(1):33–53
Girolomoni L, Cazzaniga S, Pinnola A, Perozeni F, Ballottari M, Bassi R (2019) LHCSR3 is a nonphotochemical quencher of both photosystems in Chlamydomonas reinhardtii. Proc Natl Acad Sci 116:4212–4217
He Y, Fu J, Yu C, Wang X, Jiang Q, Hong J, Lu K, Xue G, Yan C, James A (2015) Increasing cyclic electron flow is related to Na+ sequestration into vacuoles for salt tolerance in soybean. J Exp Bot 66(21):6877–6889
Hertle AP, Blunder T, Wunder T, Pesaresi P, Pribil M, Armbruster U, Leister D (2013) PGRL1 is the elusive ferredoxin-plastoquinone reductase in photosynthetic cyclic electron flow. Mol Cell 49:511–523
Horton P, Park K-J, Obayashi T, Fujita N, Harada H, Adams-Collier C, Nakai K (2007) WoLF PSORT: protein localization predictor. Nucleic Acids Res 35(2):W585–W587
Hu W, Song X, Shi K, Xia X, Zhou Y, Yu J (2008) Changes in electron transport, superoxide dismutase and ascorbate peroxidase isoenzymes in chloroplasts and mitochondria of cucumber leaves as influenced by chilling. Photosynthetica 46(4):581
Huang F, Chen K-S, Yip H-L, Hau SK, Acton O, Zhang Y, Luo J, Jen AK-Y (2009) Development of new conjugated polymers with donor− π-bridge− acceptor side chains for high performance solar cells. J Am Chem Soc 131(39):13886–13887
Huang W, Yang S-J, Zhang S-B, Zhang J-L, Cao K-F (2012) Cyclic electron flow plays an important role in photoprotection for the resurrection plant Paraboea rufescens under drought stress. Planta 235(4):819–828
Huang W, Yang Y-J, Hu H, Zhang S-B (2016) Seasonal variations in photosystem I compared with photosystem II of three alpine evergreen broad-leaf tree species. J Photochem Photobiol B 165:71–79
Huang W, Zhang S-B, Xu J-C, Liu T (2017) Plasticity in roles of cyclic electron flow around photosystem I at contrasting temperatures in the chilling-sensitive plant Calotropis gigantea. Environ Exp Bot 141:145–153
Hüner N, Dahal K, Hollis L, Bode R, Rosso D, Krol M, Ivanov AG (2012) Chloroplast redox imbalance governs phenotypic plasticity: the “grand design of photosynthesis” revisited. Front Plant Sci 3:255
Ivanov AG, Morgan RM, Gray GR, Velitchkova MY, Hüner NP (1998) Temperature/light dependent development of selective resistance to photoinhibition of photosystem I. FEBS Lett 430(3):288–292
Iwai M, Takizawa K, Tokutsu R, Okamuro A, Takahashi Y, Minagawa J (2010) Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis. Nature 464(7292):1210–1213
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophyll a, b, c1, c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Julkowska M (2020) Extreme Engineering: How Antarctic Algae Adapt to Hypersalinity. Plant Physiol 183(2):427
Kalra I, Wang X, Cvetkovska M, Jeong J, McHargue W, Zhang R, Hüner N, Yuan JS, Morgan-Kiss R (2020) Chlamydomonas sp. UWO 241 Exhibits high cyclic electron flow and rewired metabolism under high salinity. Plant Physiol 183(2):588–601
Kovács L, Vidal-Meireles A, Nagy V, Tóth SZ (2016) Quantitative determination of ascorbate from the green alga Chlamydomonas reinhardtii by HPLC. Bio Protocols 6:2067
Kramer DM, Evans JR (2011) The importance of energy balance in improving photosynthetic efficiency. Plant Physiol 155:70–78
Kramer DM, Johnson G, Kiirats O, Edwards GE (2004) New fluorescence parameters for the determination of Qa redox state and excitation energy fluxes. Photosynth Res 79(2):209–218
Kukuczka B, Magneschi L, Petroutsos D, Steinbeck J, Bald T, Powikrowska M, Fufezan C, Finazzi G, Hippler M (2014) Proton gradient regulation5-like1-mediated cyclic electron flow is crucial for acclimation to anoxia and complementary to nonphotochemical quenching in stress adaptation. Plant Physiol 165:1604–1617
Ledford HK, Chin BL, Niyogi KK (2007) Acclimation to singlet oxygen stress in Chlamydomonas reinhardtii. Eukaryot Cell. https://doi.org/10.1128/ec.00207-06LB-Ledford2007
Liefer JD, Garg A, Campbell DA, Irwin AJ, Finkel ZV (2018) Nitrogen starvation induces distinct photosynthetic responses and recovery dynamics in diatoms and Prasinophytes. PLoS ONE 13:1–24
Liu Y, Qi M, Li T (2012) Photosynthesis, photoinhibition, and antioxidant system in tomato leaves stressed by low night temperature and their subsequent recovery. Plant Sci 196:8–17
Lucker B, Kramer DM (2013) Regulation of cyclic electron flow in Chlamydomonas reinhardtii under fluctuating carbon availability. Photosynth Res 117(1–3):449–459
Maruta T, Ishikawa T (2017) Ascorbate peroxidases: crucial roles of antioxidant enzymes in plant stress responses. In: Hossain MA (ed) Ascorbic acid in plant growth, development and stress tolerance. Springer, Berlin, pp 111–127
Maruyama S, Tokutsu R, Minagawa J (2014) Transcriptional regulation of the stress-responsive light harvesting complex genes in Chlamydomonas reinhardtii. Plant Cell Physiol 55:1304–1310
Maxwell DP, Falk S, Trick CG, Huner NPA (1994) Growth a low temperature mimics high-light acclimation in Chlorella vulgaris. Plant Physiol 105:535–543
McNeill J, Barrie F, Buck W, Demoulin V, Greuter W, Hawksworth D, Herendeen P, Knapp S, Marhold K, Prado J (2012) International Code of Nomenclature for algae, fungi and plants. Regnum Vegetabile 154
Minagawa J (2011) State transitions—the molecular remodeling of photosynthetic supercomplexes that controls energy flow in the chloroplast. Biochim Et Biophys Acta-Bioenerg 1807(8):897–905
Møller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Morgan-Kiss R, Ivanov AG, Williams J, Mobashsher K, Hüner NP (2002a) Differential thermal effects on the energy distribution between photosystem II and photosystem I in thylakoid membranes of a psychrophilic and a mesophilic alga. Biochim Biophys Acta 1561(2):251–265
Morgan-Kiss RM, Ivanov AG, Hüner NPA (2002b) The Antarctic psychrophile, Chlamydomonas subcaudata, is deficient in state I-state II transitions. Planta 214(3):435–445
Morgan-Kiss RM, Ivanov AG, Modla S, Czymmek K, Hüner NP, Priscu JC, Lisle JT, Hanson TE (2008) Identity and physiology of a new psychrophilic eukaryotic green alga, Chlorella sp, strain BI, isolated from a transitory pond near Bratina Island, Antarctica. Extremophiles 12(5):701–711
Morgan-Kiss RM, Priscu JC, Pocock T, Gudynaite-Savitch L, Huner NP (2006) Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol Mol Biol Rev 70(1):222–252
Morgan RM, Ivanov AG, Priscu JC, Maxwell DP, Hüner NPA (1998) Structure and composition of the photochemical apparatus of the Antarctic green alga, Chlamydomonas subcaudata. Photosyn Res 56:303–314
Mou S, Zhang X, Ye N, Dong M, Liang C, Liang Q, Miao J, Xu D, Zheng Z (2012) Cloning and expression analysis of two different LhcSR genes involved in stress adaptation in an Antarctic microalga Chlamydomonas Sp ICE-l. Extremophiles 16:193–203
Müller P, Li X-P, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125(4):1558–1566
Neale PJ, Priscu JC (1995) The photosynthetic apparatus of phytoplankton from a perennially ice-covered Antarctic lake: acclimation to an extreme shade environment. Plant Cell Physiol 36:253–263
Nichols HW, Bold HC (1965) Trichosarcina polymorpha Gen. Et Sp Nov J Phycol 1:34–38
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Biol 50(1):333–359
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol 49(1):249–279
Öquist G, Huner NP (2003) Photosynthesis of overwintering evergreen plants. Annu Rev Plant Biol 54:329–355
Peers G, Truong TB, Ostendorf E, Busch A, Elrad D, Grossman AR, Hippler M, Niyogi KK (2009) An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462(7272):518–521
Pérez-Pérez ME, Couso I, Crespo JL (2012) Carotenoid deficiency triggers autophagy in the model green alga Chlamydomonas reinhardtii. Autophagy 8(3):376–388
Pitsch NT, Witsch B, Baier M (2010) Comparison of the chloroplast peroxidase system in the chlorophyte Chlamydomonas reinhardtii, the bryophyte Physcomitrella patens, the lycophyte Selaginella moellendorffii and the seed plant Arabidopsis thaliana. BMC Plant Biol 10(1):133
Pocock T (2004) Phylogeny, photoinhibition and recovery of a new Antarctic psychrophile Chlamydomonas raudensis (UWO 241). Ph.D., University of Western Ontario, London
Pocock T, Koziak A, Rosso D, Falk S, Huner HPA (2007) Chlamydomonas raudensis ettl. (UWO241) exhibits the capacity for rapid D1 repair in response to chronic photoinhibition at low temperature. J Phycol 43:924–936
Pocock T, Vetterli A, Falk S (2011) Evidence for phenotypic plasticity in the Antarctic extremophile Chlamydomonas raudensis Ettl. UWO 241. J Exp Bot 62(3):1169–1177. https://doi.org/10.1093/jxb/erq347
Possmayer M, Gupta RK, Szyszka-Mroz B, Maxwell DP, Lachance MA, Hüner N, Smith DR (2016) Resolving the phylogenetic relationship between Chlamydomonas sp. UWO 241 and Chlamydomonas raudensis SAG 49.72 (Chlorophyceae) with nuclear and plastid DNA sequences. J Phycol 52(2):305–310
Raymond JA, Morgan-Kiss R (2013) Separate origins of ice-binding proteins in Antarctic Chlamydomonas species. PLoS ONE 8(3):e59186
Raymond JA, Morgan-Kiss R, Stahl-Rommel S (2020) Glycerol is an osmoprotectant in two antarctic Chlamydomonas species from an ice-covered saline lake and is synthesized by an unusual bidomain enzyme. Front Plant Sci 11:1259
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:217037
Sievers F, Higgins DG (2018) Clustal omega for making accurate alignments of many protein sequences. Protein Sci 27(1):135–145
Sirikhachornkit A, Niyogi KK (2010) Antioxidants and photo-oxidative stress responses in plants and algae. In: Govindjee X, Sharkey TD (eds) Advances in photosynthesis and respiration, vol 31. Springer, Dordrecht, pp 379–396
Smith BM, Morrissey PJ, Guenther JE, Nemson JA, Harrison MA, Allen JF, Melis A (1990) Response of the photosynthetic apparatus in Dunaliella salina (green algae) to irradiance stress. Plant Physiol 93(4):1433–1440
Strand DD, Livingston AK, Satoh-Cruz M, Froehlich JE, Kramer MVG (2015) Activation of cyclic electron flow by hydrogen peroxide in vivo. Proc Natl Acad Sci 112:5539–5544
Strzepek RF, Boyd PW, Sunda WG (2019) Photosynthetic adaptation to low iron, light, and temperature in Southern Ocean phytoplankton. Proc Natl Acad Sci USA 116:4388–4393
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35(2):259–270
Szyszka-Mroz B, Cvetkovska M, Ivanov AG, Smith DR, Possmayer M, Maxwell DP, Hüner NP (2019) Cold-adapted protein kinases and thylakoid remodeling impact energy distribution in an Antarctic psychrophile. Plant Physiol 180(3):1291–1309
Szyszka-Mroz B, Pittock P, Ivanov AG, Lajoie G, Hüner NP (2015) The Antarctic psychrophile, Chlamydomonas sp. UWO 241, preferentially phosphorylates a PSI-cytochrome b6/f supercomplex. Plant Physiol 169:717–736
Szyszka B, Ivanov AG, Hüner NP (2007) Psychrophily is associated with differential energy partitioning, photosystem stoichiometry and polypeptide phosphorylation in Chlamydomonas raudensis. Biochim Biophys Acta BBA (BBA) Bioenerg 1767(6):789–800
Takahashi S, Murata N (2008) How do environmental stresses accelerate photoinhibition? Trends Plant Sci 13(4):178–182
Takahashi H, Clowez S, Wollman FA, Vallon O, Rappaport F (2013) Cyclic electron flow is redox-controlled but independent of state transition. Nat Commun 4:1–8
Tanaka A, Melis A (1997) Irradiance-dependent changes in the size and composition of the chlorophyll a-b light-harvesting complex in the green alga Dunaliella salina. Plant Cell Physiol 38(1):17–24
Tardif M, Atteia A, Specht M, Cogne G, Rolland N, Brugière S, Hippler M, Ferro M, Bruley C, Peltier G (2012) PredAlgo: a new subcellular localization prediction tool dedicated to green algae. Mol Biol Evol 29(12):3625–3639
Teixeira FK, Menezes-Benavente L, Margis R, Margis-Pinheiro M (2004) Analysis of the molecular evolutionary history of the ascorbate peroxidase gene family: inferences from the rice genome. J Mol Evol 59(6):761–770
Van Alstyne KL, Sutton L, Gifford S-A (2020) Inducible versus constitutive antioxidant defenses in algae along an environmental stress gradient. Mar Ecol Prog Ser 640:107–115
Velitchkova M, Popova AV, Faik A, Gerganova M, Ivanov AG (2020) Low temperature and high light dependent dynamic photoprotective strategies in Arabidopsis thaliana. Physiol Plant 170:93–108
Venisse J-S, Gullner G, Brisset M-N (2001) Evidence for the involvement of an oxidative stress in the initiation of infection of pear by Erwinia amylovora. Plant Physiol 125(4):2164–2172
Wildi B, Lütz C (1996) Antioxidant composition of selected high alpine plant species from different altitudes. Plant Cell Environ 19(2):138–146
Wilson KE, Hüner NP (2000) The role of growth rate, redox-state of the plastoquinone pool and the trans-thylakoid DpH in photoacclimation of Chlorella vulgaris to growth irradiance and temperature. Planta 212(1):93–102
Witman GB (1993) Chlamydomonas phototaxis. Trends Cell Biol 3(11):403–408
Wu B, Wang B (2019) Comparative analysis of ascorbate peroxidases (APXs) from selected plants with a special focus on Oryza sativa employing public databases. PLoS ONE 14(12):10226543
Yamori W, Makino A, Shikanai T (2016) A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci Rep 6:20147
Young JN, Schmidt K (2020) Its whats inside that matters: physiological adaptations of high-latitude marine microalgae to environmental change. New Phytol 5:1307–1318
Zechmann B, Stumpe M, Mauch F (2011) Immunocytochemical determination of the subcellular distribution of ascorbate in plants. Planta 233(1):1–12
Zhang C, Liu J, Zhang Y, Cai X, Gong P, Zhang J, Wang T, Li H, Ye Z (2011) Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Cell Rep 30(3):389–398
Zhang S, Scheller HV (2004) Photoinhibition of photosystem I at chilling temperature and subsequent recovery in Arabidopsis thaliana. Plant Cell Physiol 45(11):1595–1602
Zhang X, Cvetkovska M, Morgan-Kiss R, Hüner NP, Smith DR (2021) Draft genome sequence of the Antarctic green alga Chlamydomonas sp. UWO241. Iscience 24(2):102084
Zheng Y, Xue C, Chen H, He C, Wang Q (2020) Low-temperature adaptation of the snow alga Chlamydomonas nivalis is associated with the photosynthetic system regulatory process. Front Microbiol 11:1233
Acknowledgements
The authors thank Prof. John C. Priscu (Montana State University) for isolation and donation of the algal strain Chlamydomonas sp. UWO 241 (originally named Chlamydomonas subcaudata) from Lake Bonney, McMurdo Dry Valleys, Antarctica. RMK, SS-R, and MH were supported by the National Science Foundation, Office of Polar Programs under Award #OPP-1056396 (growth physiology, photoinhibition, antioxidant measurements). IK, SD and DP were supported by the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences (BES) under award #DE-SC0019138 (PSI, CEF measurements). MC was supported by a National Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant RGPIN-2019-05763 and University of Ottawa start-up funding (genome screening).
Funding
RMK, SS-R, and MH were supported by the National Science Foundation, Office of Polar Programs under Award #OPP-1056396 (growth physiology, photoinhibition, antioxidant measurements). IK, SD and DP were supported by the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences (BES) under Award #DE-SC0019138 (PSI, CEF, H2O2 measurements). MC is supported by a National Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant RGPIN-2019–05763 and University of Ottawa start-up funding (genome screening).
Author information
Authors and Affiliations
Contributions
SS-R and RMK conceptualized the research; SS-R, SD, IK, MH, DP, and MC performed the investigations; SS-R, RMK, and MH developed the methodology; SS-R, RMK and MC performed data curation; RMK provided project administration; RMK and MC helped with funding acquisition; SS-R and RMK wrote the original draft of the manuscript; SD, IK, MH, and MC reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stahl-Rommel, S., Kalra, I., D’Silva, S. et al. Cyclic electron flow (CEF) and ascorbate pathway activity provide constitutive photoprotection for the photopsychrophile, Chlamydomonas sp. UWO 241 (renamed Chlamydomonas priscuii). Photosynth Res 151, 235–250 (2022). https://doi.org/10.1007/s11120-021-00877-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-021-00877-5