Abstract
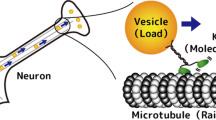
The cells of all living creatures rely on a host of molecular scale machines to perform vital tasks. In the spirit of this special issue of J. Stat. Phys., we describe briefly the background concerning one class of these machines, namely, processive motor proteins such as, specifically, conventional kinesin, myosin V, and cytoplasmic dynein. These single-molecule motors tow cellular cargoes under load along oriented linear molecular tracks within the cell taking many hundreds of consecutive discrete steps. Experiments aimed at understanding the mechanism of the stepping process have recently led to observations of ‘‘limping” in which alternate steps are found to be slow or fast, respectively. Reliable experimental measurements of the ‘‘true” or intrinsic limping factor, L 0, understood as the ideal overall ratio of the longer dwell times prior to one set of steps to the shorter times for the interlaced steps, provide a route to improving appropriate biomechanochemical models. These, in turn, may help reveal and quantify details of the underlying asymmetric walking mechanisms. However, a difficulty is posed in measuring L 0 by the inescapable thermal fluctuations that act on an individual motor molecule that takes only a finite number, say, n odd and n even steps under fixed load, etc. Accordingly, we treat the stochastic issues theoretically for some basic kinetic motor models and experimental procedures, obtaining various exact bounds and explicit results for distributions and their moments. Typically for n≲10 the observed mean values, 〈L n 〉, significantly overestimate L 0. However, the medians and rescaled means, \({\overline{L}}_{n}^{\, *}=\langle L_{n}\rangle(n-1)/n\), provide better guides to the value of L 0 provided it is not too close to unity. Separately, we present figures, a table, and approximate formulas intended to assist practically those designing, undertaking, and assessing experiments on limping.
Similar content being viewed by others
References
Amos, L.A., Klug, A.: Arrangement of subunits in flagellar microtubules. Proc. Natl. Acad. Sci. USA 14, 523–549 (1974)
Asbury, C.L.: Kinesin: world’s tiniest biped. Curr. Opin. Cell Biol. 17, 89–97 (2005)
Asbury, C.L., Fehr, A.N., Block, S.M.: Kinesin moves by an asymmetric hand-over-hand mechanism. Science 302, 2130–2134 (2003)
Block, S.M.: Kinesin motor mechanics: Binding, stepping, tracking, gating, and limping. Biophys. J. 92, 2986–2995 (2007)
Block, S.M., Goldstein, L.S.B., Schnapp, B.J.: Bead movement by single kinesin molecules studied with optical tweezers. Nature 348, 348–352 (1990)
Block, S.M., Asbury, C.L., Shaevitz, J.W., Lang, M.J.: Probing the kinesin reaction cycle with a 2D optical force clamp. Proc. Natl. Acad. Sci. USA 100, 2351–2356 (2003)
Bray, D.: Cell Movements: From Molecules to Motility, 2nd edn. Garland, New York (2001)
Carter, N.J., Cross, R.A.: Mechanics of the kinesin step. Nature 435, 308–312 (2005)
Cooper, G.M.: The Cell: A Molecular Approach, 2nd edn. Sinauer Associates, Sunderland (2000)
Coppin, C.M., Finer, J.T., Spudich, J.A., Vale, R.D.: Detection of sub-8-nm movements of kinesin by high-resolution optical-trap microscopy. Proc. Natl. Acad. Sci. USA 93, 1913–1917 (1996)
Coppin, C.M., Pierce, D.W., Hsu, L., Vale, R.D.: The load dependence of kinesin’s mechanical cycle. Proc. Natl. Acad. Sci. USA 94, 8539–8544 (1997)
Coy, D.L., Wagenbach, M., Howard, J.: Kinesin takes one 8-nm step for each ATP that it hydrolyzes. J. Biol. Chem. 274, 3667–3671 (1999)
Cross, R.A.: Molecular motors: Kinesin’s interesting limp. Curr. Biol. 14, R158–R159 (2004)
Dagenbach, E.M., Endow, S.A.: A new kinesin tree. J. Cell. Sci. 117, 3–7 (2004)
Derrida, B.: Velocity and diffusion constant of a periodic one-dimensional hopping model. J. Stat. Phys. 31, 433–450 (1983)
Erdélyi, A.: Higher Transendental Functions. Bateman Manuscript Project, vol. I & II. McGraw-Hill, New York (1953)
Fehr, A.N., Asbury, C.L., Block, S.M.: Kinesin steps do not alternate in size. Biophys. J. 94, L20–L22 (2008)
Fehr, A.N., Gutiérrez-Medina, B., Asbury, C.L., Block, S.M.: On the origin of kinesin limping. Biophys. J. 97, 1663–1670 (2009)
Fisher, M.E., Kim, Y.C.: Kinesin crouches to sprint but resists pushing. Proc. Natl. Acad. Sci. USA 102, 16209–16214 (2005)
Fisher, M.E., Kolomeisky, A.B.: Molecular motors and the forces they exert. Physica A 274, 241–266 (1999)
Fisher, M.E., Kolomeisky, A.B.: Simple mechanochemistry describes the dynamics of kinesin molecules. Proc. Natl. Acad. Sci. USA 98, 7748–7753 (2001)
Greenleaf, W.J., Woodside, M.T., Block, S.M.: High-resolution, single-molecule measurements of biomolecular motion. Annu. Rev. Biophys. Biomol. Struct. 36, 171–190 (2007)
Guydosh, N.R., Block, S.M.: Backsteps induced by nucleotide analogs suggest the front head of kinesin is gated by strain. Proc. Natl. Acad. Sci. USA 103, 8054–8059 (2006)
Guydosh, N.R., Block, S.M.: Not so lame after all: Kinesin still walks with a hobbled head. J. Gen. Physiol. 130, 441–444 (2007)
Hackney, D.D.: Processive motor movement. Science 316, 58–59 (2007)
Higuchi, H., Muto, E., Inoue, Y., Yanagida, T.: Kinetics of force generation by single kinesin molecules activated by laser photolysis of caged ATP. Proc. Natl. Acad. Sci. USA 94, 4395–4400 (1997)
Higuchi, H., Bronner, C.E., Park, H.W., Endow, S.A.: Rapid double 8-nm steps by a kinesin mutant. EMBO J. 23, 2993–2999 (2004)
Houdusse, A., Carter, A.P.: Dynein swings into action. Cell 136, 395–396 (2009)
Howard, J.: Mechanics of Motor Proteins and the Cytoskeleton. Sinauer Associates, Sunderland (2001)
Hua, W., Young, E.C., Fleming, M.L., Gelles, J.: Coupling of kinesin steps to ATP hydrolysis. Nature 388, 390–393 (1997)
Hyeon, C., Klumpp, S., Onuchic, J.N.: Kinesin’s backsteps under mechanical load. Phys. Chem. Chem. Phys. 11, 4899–4910 (2009)
Kaseda, K., Higuchi, H., Hirose, K.: Alternate fast and slow stepping of a heterodimeric kinesin molecule. Nat. Cell Biol. 23, 1079–1082 (2003)
Kim, Y.C., Fisher, M.E.: Vectorial loading of processive motor proteins: implementing a landscape picturer. J. Phys., Condens. Matter 17, S3821–S3838 (2005)
Kojima, H., Muto, E., Higuchi, H., Yanagida, T.: Mechanics of single kinesin molecules measured by optical trapping nanometry. Biophys. J. 73, 2012–2022 (1997)
Kolomeisky, A.B., Fisher, M.E.: Extended kinetic models with waiting-time distributions: Exact results. J. Chem. Phys. 113, 10867 (2000)
Kolomeisky, A.B., Fisher, M.E.: Periodic sequential kinetic models with jumping, branching and deaths. Physica A 279, 1–20 (2000)
Kolomeisky, A.B., Fisher, M.E.: Molecular motors: a theorist’s perspective. Annu. Rev. Phys. Chem. 58, 675–695 (2007)
Kolomeisky, A.B., Stukalin, E.B., Popov, A.A.: Understanding mechanochemical coupling in kinesins using first-passage-time processes. Phys. Rev. E 71, 031902 (2005)
Lang, M.J., Asbury, C.L., Shaevitz, J.W., Block, S.M.: An automated two-dimensional optical force clamp for single molecule studies. Biophys. J. 83, 491–501 (2002)
Mallik, R., Carter, B.C., Lex, S.A., King, S.J., Gross, S.P.: Cytoplasmic dynein functions as a gear in response to load. Nature 427, 649–652 (2004)
Mandelkow, E., Thomas, J., Cohen, C.: Microtubule structure at low resolution by x-ray diffraction. Proc. Natl. Acad. Sci. USA 74, 3370–3374 (1977)
Mehta, A.D., Rock, R.S., Rief, M., Spudich, J.A., Mooseker, M.S., Cheney, R.E.: Myosin-V is a processive actin-based motor. Nature 400, 590–593 (1999)
Meyhöfer, E., Howard, J.: The force generated by a single kinesin molecule against an elastic load. Proc. Natl. Acad. Sci. USA 92, 574–578 (1995)
Nishiyama, M., Higuchi, H., Yanagida, T.: Chemomechanical coupling of the forward and backward steps of single kinesin molecules. Nat. Cell Biol. 4, 790–797 (2002)
Ökten, Z., Churchman, L.S., Rock, R.S., Spudich, J.A.: Myosin VI walks hand-over-hand along actin. Nat. Struct. Mol. Biol. 11, 884–887 (2004)
Ou-Yang, H.D., Wei, M.T.: Complex fluids: Probing mechanical properties of biological systems with optical tweezers. Annu. Rev. Phys. Chem. 61, 421–440 (2010)
Redner, S.: A Guide to First-Passage Processes. Cambridge University Press, Cambridge (2001)
Reimann, P.: Brownian motors: noisy transport far from equilibrium. Phys. Rep. 361, 57–265 (2002)
Roberts, A.J., Numata, N., Walker, M.L., Kato, Y.S., Malkova, B., Kon, T., Ohkura, R., Arisaka, F., Knight, P.J., Sutoh, K., Burgess, S.A.: AAA+ ring and linker swing mechanism in the dynein motor. Cell 136, 485–495 (2009)
Rock, R.S., Rice, S.E., Wells, A.L., Purcell, T.J., Spudich, J.A., Sweeney, H.L.: Myosin VI is a processive motor with a large step size. Proc. Natl. Acad. Sci. USA 98, 13655–13659 (2001)
Rosenfeld, S.S., Fordyce, P.M., Jeffersonand, G.M., King, P.H., Block, S.M.: Stepping and stretching: How kinesin uses internal strain to walk processively. J. Biol. Chem. 278, 18550–18556 (2003)
Schliwa, M.: Molecular Motors. Wiley-Vch, Weinheim (2003)
Schnitzer, M.J., Block, S.M.: Kinesin hydrolyses one ATP per 8-nm step. Nature 388, 386–390 (1997)
Snyder, G.E., Sakamoto, T., Hammer, J.A., Sellers, J.R., Selvin, P.R.: Nanometer localization of single green fluorescent proteins: Evidence that Myosin V walks hand-over-hand via telemark configuration. Biophys. J. 87, 1776–1783 (2004)
Sperry, A.O.: Molecular Motors: Methods and Protocols. Methods in Molecular Biology, vol. 392. Humana Press, Totowa (2007)
Svoboda, K., Block, S.M.: Force and velocity measured for single kinesin molecules. Cell 77, 773–784 (1994)
Thorn, K.S., Ubersax, J.A., Vale, R.D.: Engineering the processive run length of the kinesin motor. J. Cell Biol. 151, 1093–1100 (2000)
Toba, S., Watanabe, T.M., Yamaguchi-Okimoto, L., Toyoshima, Y.Y., Higuchi, H.: Overlapping hand-over-hand mechanism of single molecular motility of cytoplasmic dynein. Proc. Natl. Acad. Sci. USA 103, 5741–5745 (2006)
Toprak, E., Yildiz, A., Hoffman, M.T., Rosenfeld, S.S., Selvin, P.R.: Why kinesin is so processive. Proc. Natl. Acad. Sci. USA 106, 12717–12722 (2009)
Tsygankov, D., Fisher, M.E.: Mechanoenzymes under superstall and large assisting loads reveal structural features. Proc. Natl. Acad. Sci. USA 104, 19321–19326 (2007)
Vale, R.D.: The molecular motor toolbox for intracellular transport. Cell 112, 467–480 (2003)
Vale, R.D.: Myosin V motor proteins: marching stepwise towards a mechanism. J. Cell Biol. 163, 445–450 (2003)
van Kampen, N.G.: Stochastic Processes in Physics and Chemistry, 2nd edn. Elsevier, Amsterdam (1997) Chap. XII
Veigel, C., Molloy, J.E., Schmitz, S., Kendrick-Jones, J.: Load-dependent kinetics of force production by smooth muscle myosin measured with optical tweezers. Nat. Cell Biol. 5, 980–986 (2003)
Yildiz, A., Forkey, J.N., McKinney, S.A., Ha, T., Goldman, Y.E., Selvin, P.R.: Myosin-V walks hand-over-hand: Single fluorophore imaging with 1.5-nm localization. Science 300, 2016–2065 (2003)
Yildiz, A., Park, H., Safer, D., Yang, Z., Chen, L.Q., Selvin, P.R., Sweeney, H.L.: Myosin VI steps via a hand-over-hand mechanism with its lever arm undergoing fluctuations when attached to actin. J. Biol. Chem. 279, 37223–37226 (2004)
Yildiz, A., Tomishige, M., Vale, R.D., Selvin, P.R.: Kinesin walks hand-over-hand. Science 303, 676–678 (2004)
Yildiz, A., Tomishige, M., Gennerich, A., Vale, R.D.: Intramolecular strain coordinates kinesin stepping behavior along microtubules. Cell 134, 1030–1041 (2008)
Zhang, Y.: Derivation of diffusion coefficient of a Brownian particle in tilted periodic potential from the coordinate moments. Phys. Lett. A 373, 2629–2633 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Fisher, M.E. Measuring the Limping of Processive Motor Proteins. J Stat Phys 142, 1218–1251 (2011). https://doi.org/10.1007/s10955-011-0118-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10955-011-0118-x