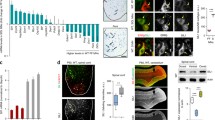
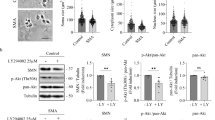
Abstract
Spinal Muscular Atrophy (SMA) is a neurodegenerative disease that is caused by deletion of the SMN (Survival of Motor Neuron) gene. The SMN protein is essential for cell survival and co-localized with TIA-1/R and G3BP, two characteristic markers of stress granules (SGs). To further study the SMN function in stress granules and in response to stress, we generated stable cell lines with SMN knockdown. Our data indicate that suppression of SMN drastically reduces cellular ability to form stress granules in response to stress treatment. In addition, we show that SMN deficiency sensitizes cells to sodium arsenite and H2O2, two well-known stress inducers, leading to cell death at a much lower concentration of inducers in SMN knockdown cells than in control cells. Interestingly, the cell death is correlated with formation of stress granules, suggesting that involvement of SMN in formation of stress granules may play an important role in cell survival. Furthermore, rescue of SGs formation by overexpression of G3BP can reverse the defective formation of stress granules and results in partial abrogation of cell death against SMN deficiency. We deduce that modulation of stress response may be useful for potential SMN treatment.





Similar content being viewed by others
References
Anderson P, Kedersha N (2002) Stressful initiations. J Cell Sci 115(Pt 16):3227–3234
Baccon J, Pellizzoni L et al (2002) Identification and characterization of Gemin7, a novel component of the survival of motor neuron complex. J Biol Chem 277(35):31957–31962
Batulan Z, Shinder GA et al (2003) High threshold for induction of the stress response in motor neurons is associated with failure to activate HSF1. J Neurosci 23(13):5789–5798
Berlanga JJ, Herrero S et al (1998) Characterization of the hemin-sensitive eukaryotic initiation factor 2alpha kinase from mouse nonerythroid cells. J Biol Chem 273(48):32340–32346
Burlet P, Huber C et al (1998) The distribution of SMN protein complex in human fetal tissues and its alteration in spinal muscular atrophy. Hum Mol Genet 7(12):1927–1933
Carissimi C, Saieva L et al (2006) Gemin8 is a novel component of the survival motor neuron complex and functions in small nuclear ribonucleoprotein assembly. J Biol Chem 281(12):8126–8134
Carrel TL, McWhorter ML et al (2006) Survival motor neuron function in motor axons is independent of functions required for small nuclear ribonucleoprotein biogenesis. J Neurosci 26(43):11014–11022
Charroux B, Pellizzoni L et al (1999) Gemin3: a novel DEAD box protein that interacts with SMN, the spinal muscular atrophy gene product, and is a component of gems. J Cell Biol 147(6):1181–1194
Charroux B, Pellizzoni L et al (2000) Gemin4. A novel component of the SMN complex that is found in both gems and nucleoli. J Cell Biol 148(6):1177–1186
Clemens MJ (2001) Initiation factor eIF2 alpha phosphorylation in stress responses and apoptosis. Prog Mol Subcell Biol 27:57–89
Coovert DD, Le TT et al (1997) The survival motor neuron protein in spinal muscular atrophy. Hum Mol Genet 6(8):1205–1214
Crawford TO, Pardo CA (1996) The neurobiology of childhood spinal muscular atrophy. Neurobiol Dis 3(2):97–110
Dey M, Trieselmann B et al (2005) PKR and GCN2 kinases and guanine nucleotide exchange factor eukaryotic translation initiation factor 2B (eIF2B) recognize overlapping surfaces on eIF2alpha. Mol Cell Biol 25(8):3063–3075
Dodds E, Dunckley MG et al (2001) Overexpressed human survival motor neurone isoforms, SMNDeltaexon7 and SMN+exon7, both form intranuclear gems but differ in cytoplasmic distribution. FEBS Lett 495(1–2):31–38
Gangwani L, Mikrut M et al (2001) Spinal muscular atrophy disrupts the interaction of ZPR1 with the SMN protein. Nat Cell Biol 3(4):376–383
Gray NK, Wickens M (1998) Control of translation initiation in animals. Annu Rev Cell Dev Biol 14:399–458
Gubitz AK, Mourelatos Z et al (2002) Gemin5, a novel WD repeat protein component of the SMN complex that binds Sm proteins. J Biol Chem 277(7):5631–5636
Hannus S, Buhler D et al (2000) The Schizosaccharomyces pombe protein Yab8p and a novel factor, Yip1p, share structural and functional similarity with the spinal muscular atrophy-associated proteins SMN and SIP1. Hum Mol Genet 9(5):663–674
Hsieh-Li HM, Chang JG et al (2000) A mouse model for spinal muscular atrophy. Nat Genet 24(1):66–70
Hua Y, Zhou J (2004a) Rpp20 interacts with SMN and is re-distributed into SMN granules in response to stress. Biochem Biophys Res Commun 314(1):268–276
Hua Y, Zhou J (2004b) Survival motor neuron protein facilitates assembly of stress granules. FEBS Lett 572(1–3):69–74
Iannaccone ST (1998) Spinal muscular atrophy. Semin Neurol 18(1):19–26
Jablonka S, Karle K et al (2006) Distinct and overlapping alterations in motor and sensory neurons in a mouse model of spinal muscular atrophy. Hum Mol Genet 15(3):511–518
Kedersha NL, Gupta M et al (1999) RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol 147(7):1431–1442
Kedersha N, Cho MR et al (2000) Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol 151(6):1257–1268
Kedersha N, Chen S et al (2002) Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol Biol Cell 13(1):195–210
Lefebvre S, Burglen L et al (1995) Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80(1):155–165
Liu Q, Dreyfuss G (1996) A novel nuclear structure containing the survival of motor neurons protein. EMBO J 15(14):3555–3565
Liu Q, Fischer U et al (1997) The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell 90(6):1013–1021
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408
Lorson CL, Strasswimmer J et al (1998) SMN oligomerization defect correlates with spinal muscular atrophy severity. Nat Genet 19(1):63–66
Manzerra P, Brown IR (1992) Expression of heat shock genes (hsp70) in the rabbit spinal cord: localization of constitutive and hyperthermia-inducible mRNA species. J Neurosci Res 31(4):606–615
Manzerra P, Brown IR (1996) The neuronal stress response: nuclear translocation of heat shock proteins as an indicator of hyperthermic stress. Exp Cell Res 229(1):35–47
McEwen E, Kedersha N et al (2005) Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J Biol Chem 280(17):16925–16933
Meister G, Buhler D et al (2000) Characterization of a nuclear 20S complex containing the survival of motor neurons (SMN) protein and a specific subset of spliceosomal Sm proteins. Hum Mol Genet 9(13):1977–1986
Meister G, Eggert C et al (2002) SMN-mediated assembly of RNPs: a complex story. Trends Cell Biol 12(10):472–478
Melki J (1997) Spinal muscular atrophy. Curr Opin Neurol 10(5):381–385
Monani UR, Sendtner M et al (2000) The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn(−/−) mice and results in a mouse with spinal muscular atrophy. Hum Mol Genet 9(3):333–339
Nover L, Scharf KD et al (1989) Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol Cell Biol 9(3):1298–1308
Pagliardini S, Giavazzi A et al (2000) Subcellular localization and axonal transport of the survival motor neuron (SMN) protein in the developing rat spinal cord. Hum Mol Genet 9(1):47–56
Paushkin S, Gubitz AK et al (2002) The SMN complex, an assemblyosome of ribonucleoproteins. Curr Opin Cell Biol 14(3):305–312
Pearn J (1980) Classification of spinal muscular atrophies. Lancet 1(8174):919–922
Pellizzoni L, Kataoka N et al (1998) A novel function for SMN, the spinal muscular atrophy disease gene product, in pre-mRNA splicing. Cell 95(5):615–624
Pellizzoni L, Baccon J et al (2002) Purification of native survival of motor neurons complexes and identification of Gemin6 as a novel component. J Biol Chem 277(9):7540–7545
Rossoll W, Kroning AK et al (2002) Specific interaction of Smn, the spinal muscular atrophy determining gene product, with hnRNP-R and gry-rbp/hnRNP-Q: a role for Smn in RNA processing in motor axons? Hum Mol Genet 11(1):93–105
Rossoll W, Jablonka S et al (2003) Smn, the spinal muscular atrophy-determining gene product, modulates axon growth and localization of beta-actin mRNA in growth cones of motoneurons. J Cell Biol 163(4):801–812
Sahin M, Greer PL et al (2005) Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron 46(2):191–204
Schrank B, Gotz R et al (1997) Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc Natl Acad Sci USA 94(18):9920–9925
Sleeman JE, Trinkle-Mulcahy L et al (2003) Cajal body proteins SMN and Coilin show differential dynamic behaviour in vivo. J Cell Sci 116(Pt 10):2039–2050
Todd AG, Shaw DJ et al (2010) SMN and the Gemin proteins form sub-complexes that localise to both stationary and dynamic neurite granules. Biochem Biophys Res Commun 394(1):211–216
Tourriere H, Chebli K et al (2003) The RasGAP-associated endoribonuclease G3BP assembles stress granules. J Cell Biol 160(6):823–831
Vyas S, Bechade C et al (2002) Involvement of survival motor neuron (SMN) protein in cell death. Hum Mol Genet 11(22):2751–2764
Wang J, Dreyfuss G (2001) A cell system with targeted disruption of the SMN gene: functional conservation of the SMN protein and dependence of Gemin2 on SMN. J Biol Chem 276(13):9599–9605
Yang J, Mani SA et al (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117(7):927–939
Young PJ, Man NT et al (2000) The exon 2b region of the spinal muscular atrophy protein, SMN, is involved in self-association and SIP1 binding. Hum Mol Genet 9(19):2869–2877
Zhan K, Vattem KM et al (2002) Phosphorylation of eukaryotic initiation factor 2 by heme-regulated inhibitor kinase-related protein kinases in Schizosaccharomyces pombe is important for resistance to environmental stresses. Mol Cell Biol 22(20):7134–7146
Zhang P, McGrath B et al (2002) The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 22(11):3864–3874
Zhang HL, Pan F et al (2003) Active transport of the survival motor neuron protein and the role of exon-7 in cytoplasmic localization. J Neurosci 23(16):6627–6637
Zhang Z, Lotti F et al (2008) SMN deficiency causes tissue-specific perturbations in the repertoire of snRNAs and widespread defects in splicing. Cell 133(4):585–600
Zou T, Ling C et al (2006) Exogenous tissue plasminogen activator enhances peripheral nerve regeneration and functional recovery after injury in mice. J Neuropathol Exp Neurol 65(1):78–86
Zufferey R, Nagy D et al (1997) Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 15(9):871–875
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zou, T., Yang, X., Pan, D. et al. SMN Deficiency Reduces Cellular Ability to Form Stress Granules, Sensitizing Cells to Stress. Cell Mol Neurobiol 31, 541–550 (2011). https://doi.org/10.1007/s10571-011-9647-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-011-9647-8