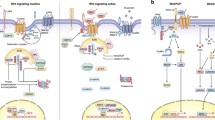
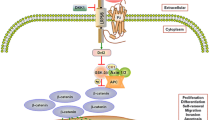
Abstract
Since the discovery of the first mammalian Wnt proto-oncogene in virus-induced mouse mammary tumors almost four decades ago, Wnt signaling pathway and its involvement in cancers have been extensively investigated. Activation of this evolutionarily conserved pathway promotes cancer development via diverse mechanisms. Cancer is a complex disease and one outstanding conceptual framework for understanding its biology is the “Hallmarks of Cancer”. In this review, we focus on the involvement of Wnt signaling in the ten hallmarks of human cancer. These widespread roles of Wnt signaling in human cancers highlight the importance and feasibility of targeting this signaling pathway for cancer treatment.



Similar content being viewed by others
References
Srivastava, M., Begovic, E., Chapman, J., Putnam, N. H., Hellsten, U., Kawashima, T., et al. (2008). The Trichoplax genome and the nature of placozoans. Nature, 454(7207), 955–960. https://doi.org/10.1038/nature07191.
Nichols, S. A., Dirks, W., Pearse, J. S., & King, N. (2006). Early evolution of animal cell signaling and adhesion genes. Proceedings of the National Academy of Sciences of the United States of America, 103(33), 12451–12456. https://doi.org/10.1073/pnas.0604065103.
Loh, K. M., van Amerongen, R., & Nusse, R. (2016). Generating cellular diversity and spatial form: Wnt signaling and the evolution of multicellular animals. Developmental Cell, 38(6), 643–655. https://doi.org/10.1016/j.devcel.2016.08.011.
Nusse, R., & Varmus, H. E. (1982). Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell, 31(1), 99–109.
Aoki, K., & Taketo, M. M. (2007). Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. Journal of Cell Science, 120(Pt 19), 3327–3335. https://doi.org/10.1242/jcs.03485.
Yu, J., & Virshup, D. M. (2014). Updating the Wnt pathways. Bioscience Reports, 34(5). https://doi.org/10.1042/BSR20140119.
Steinhart, Z., & Angers, S. (2018). Wnt signaling in development and tissue homeostasis. Development, 145(11), dev146589. https://doi.org/10.1242/dev.146589.
Hanahan, D., & Weinberg, R. A. (2000). The hallmarks of cancer. Cell, 100(1), 57–70. https://doi.org/10.1016/s0092-8674(00)81683-9.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell, 144(5), 646–674. https://doi.org/10.1016/j.cell.2011.02.013.
Obaya, A. J., & Sedivy, J. M. (2002). Regulation of cyclin-Cdk activity in mammalian cells. Cellular and Molecular Life Sciences, 59(1), 126–142. https://doi.org/10.1007/s00018-002-8410-1.
Massagué, J. (2004). G1 cell-cycle control and cancer. Nature, 432(7015), 298–306. https://doi.org/10.1038/nature03094.
Niehrs, C., & Acebron, S. P. (2012). Mitotic and mitogenic Wnt signalling. The EMBO Journal, 31(12), 2705–2713. https://doi.org/10.1038/emboj.2012.124.
He, T. C., Sparks, A. B., Rago, C., Hermeking, H., Zawel, L., da Costa, L. T., et al. (1998). Identification of c-MYC as a target of the APC pathway. Science, 281(5382), 1509–1512. https://doi.org/10.1126/science.281.5382.1509.
Tetsu, O., & McCormick, F. (1999). Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature, 398(6726), 422–426. https://doi.org/10.1038/18884.
Shtutman, M., Zhurinsky, J., Simcha, I., Albanese, C., D'Amico, M., Pestell, R., & Ben-Ze'ev, A. (1999). The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proceedings of the National Academy of Sciences of the United States of America, 96(10), 5522–5527. https://doi.org/10.1073/pnas.96.10.5522.
Diehl, J. A., Cheng, M., Roussel, M. F., & Sherr, C. J. (1998). Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes & Development, 12(22), 3499–3511. https://doi.org/10.1101/gad.12.22.3499.
Welcker, M., Singer, J., Loeb, K. R., Grim, J., Bloecher, A., Gurien-West, M., et al. (2003). Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation. Molecular Cell, 12(2), 381–392. https://doi.org/10.1016/s1097-2765(03)00287-9.
Welcker, M., Orian, A., Jin, J., Grim, J. E., Grim, J. A., Harper, J. W., et al. (2004). The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proceedings of the National Academy of Sciences of the United States of America, 101(24), 9085–9090. https://doi.org/10.1073/pnas.0402770101.
Acebron, S. P., Karaulanov, E., Berger, B. S., Huang, Y.-L., & Niehrs, C. (2014). Mitotic wnt signaling promotes protein stabilization and regulates cell size. Molecular Cell, 54(4), 663–674. https://doi.org/10.1016/j.molcel.2014.04.014.
Reya, T., & Clevers, H. (2005). Wnt signalling in stem cells and cancer. Nature, 434(7035), 843–850. https://doi.org/10.1038/nature03319.
Leung, C., Tan, S. H., & Barker, N. (2018). Recent advances in Lgr5+ stem cell research. Trends in Cell Biology, 28(5), 380–391. https://doi.org/10.1016/j.tcb.2018.01.010.
Orford, K. W., & Scadden, D. T. (2008). Deconstructing stem cell self-renewal: Genetic insights into cell-cycle regulation. Nature reviews Genetics, 9(2), 115–128. https://doi.org/10.1038/nrg2269.
Kabiri, Z., Greicius, G., Zaribafzadeh, H., Hemmerich, A., Counter, C. M., & Virshup, D. M. (2018). Wnt signaling suppresses MAPK-driven proliferation of intestinal stem cells. The Journal of Clinical Investigation, 128(9). https://doi.org/10.1172/JCI99325.
Riemer, P., Sreekumar, A., Reinke, S., Rad, R., Schäfer, R., Sers, C., et al. (2015). Transgenic expression of oncogenic BRAF induces loss of stem cells in the mouse intestine, which is antagonized by β-catenin activity. Oncogene, 34(24), 3164–3175. https://doi.org/10.1038/onc.2014.247.
Zhong, Z., & Virshup, D. M. (2020). Wnt signaling and drug resistance in cancer. Molecular Pharmacology, 97(2), 72–89. https://doi.org/10.1124/mol.119.117978.
Proffitt, K. D., Madan, B., Ke, Z., Pendharkar, V., Ding, L., Lee, M. A., et al. (2013). Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Research, 73(2), 502–507. https://doi.org/10.1158/0008-5472.CAN-12-2258.
Koo, B.-K., van Es, J. H., van den Born, M., & Clevers, H. (2015). Porcupine inhibitor suppresses paracrine Wnt-driven growth of Rnf43;Znrf3-mutant neoplasia. Proceedings of the National Academy of Sciences of the United States of America, 201508113. https://doi.org/10.1073/pnas.1508113112.
Boulter, L., Guest, R. V., Kendall, T. J., Wilson, D. H., Wojtacha, D., Robson, A. J., et al. (2015). WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. The Journal of Clinical Investigation, 125(3), 1269–1285. https://doi.org/10.1172/JCI76452.
Storm, E. E., Durinck, S., de Sousa e Melo, F., Tremayne, J., Kljavin, N., Tan, C., et al. (2016). Targeting PTPRK-RSPO3 colon tumours promotes differentiation and loss of stem-cell function. Nature, 529(7584), 97–100. https://doi.org/10.1038/nature16466.
Madan, B., Harmston, N., Nallan, G., Montoya, A., Faull, P., Petretto, E., & Virshup, D. M. (2018). Temporal dynamics of Wnt-dependent transcriptome reveal an oncogenic Wnt/MYC/ribosome axis. The Journal of Clinical Investigation, 128(12), 5620–5633. https://doi.org/10.1172/JCI122383.
Lin, C. Y., Lovén, J., Rahl, P. B., Paranal, R. M., Burge, C. B., Bradner, J. E., et al. (2012). Transcriptional amplification in tumor cells with elevated c-Myc. Cell, 151(1), 56–67. https://doi.org/10.1016/j.cell.2012.08.026.
Nie, Z., Hu, G., Wei, G., Cui, K., Yamane, A., Resch, W., et al. (2012). c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell, 151(1), 68–79. https://doi.org/10.1016/j.cell.2012.08.033.
Bretones, G., Delgado, M. D., & León, J. (2015). Myc and cell cycle control. Biochimica et Biophysica Acta, 1849(5), 506–516. https://doi.org/10.1016/j.bbagrm.2014.03.013.
van Riggelen, J., Yetil, A., & Felsher, D. W. (2010). MYC as a regulator of ribosome biogenesis and protein synthesis. Nature Reviews. Cancer, 10(4), 301–309. https://doi.org/10.1038/nrc2819.
Dang, C. V. (2013). MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harbor Perspectives in Medicine, 3(8), a014217–a014217. https://doi.org/10.1101/cshperspect.a014217.
Wahlström, T., & Henriksson, M. A. (2015). Impact of MYC in regulation of tumor cell metabolism. Biochimica et Biophysica Acta, 1849(5), 563–569. https://doi.org/10.1016/j.bbagrm.2014.07.004.
Sansom, O. J., Meniel, V. S., Muncan, V., Phesse, T. J., Wilkins, J. A., Reed, K. R., et al. (2007). Myc deletion rescues Apc deficiency in the small intestine. Nature, 446(7136), 676–679. https://doi.org/10.1038/nature05674.
Meyers, R. M., Bryan, J. G., McFarland, J. M., Weir, B. A., Sizemore, A. E., Xu, H., et al. (2017). Computational correction of copy number effect improves specificity of CRISPR–Cas9 essentiality screens in cancer cells. Nature Genetics, 49(12), 1779–1784. https://doi.org/10.1038/ng.3984.
McDonald, E. R., de Weck, A., Schlabach, M. R., Billy, E., Mavrakis, K. J., Hoffman, G. R., et al. (2017). Project DRIVE: a compendium of cancer dependencies and synthetic lethal relationships uncovered by large-scale, deep RNAi screening. Cell, 170(3), 577–592.e10. https://doi.org/10.1016/j.cell.2017.07.005.
Behan, F. M., Iorio, F., Picco, G., Gonçalves, E., Beaver, C. M., Migliardi, G., et al. (2019). Prioritization of cancer therapeutic targets using CRISPR-Cas9 screens. Nature, 31, 1806. https://doi.org/10.1038/s41586-019-1103-9.
Fong, C. Y., Gilan, O., Lam, E. Y. N., Rubin, A. F., Ftouni, S., Tyler, D., et al. (2015). BET inhibitor resistance emerges from leukaemia stem cells. Nature, 525(7570), 538–542. https://doi.org/10.1038/nature14888.
Rathert, P., Roth, M., Neumann, T., Muerdter, F., Roe, J.-S., Muhar, M., et al. (2015). Transcriptional plasticity promotes primary and acquired resistance to BET inhibition. Nature, 525(7570), 543–547. https://doi.org/10.1038/nature14898.
Harbour, J. W., & Dean, D. C. (2000). Rb function in cell-cycle regulation and apoptosis. Nature Cell Biology, 2(4), E65–E67. https://doi.org/10.1038/35008695.
Giacinti, C., & Giordano, A. (2006). RB and cell cycle progression. Oncogene, 25(38), 5220–5227. https://doi.org/10.1038/sj.onc.1209615.
Henley, S. A., & Dick, F. A. (2012). The retinoblastoma family of proteins and their regulatory functions in the mammalian cell division cycle. Cell Division, 7(1), 10–14. https://doi.org/10.1186/1747-1028-7-10.
Ohtani, K., DeGregori, J., & Nevins, J. R. (1995). Regulation of the cyclin E gene by transcription factor E2F1. Proceedings of the National Academy of Sciences of the United States of America, 92(26), 12146–12150. https://doi.org/10.1073/pnas.92.26.12146.
Bracken, A. P., Ciro, M., Cocito, A., & Helin, K. (2004). E2F target genes: unraveling the biology. Trends in Biochemical Sciences, 29(8), 409–417. https://doi.org/10.1016/j.tibs.2004.06.006.
Delmas, V., Beermann, F., Martinozzi, S., Carreira, S., Ackermann, J., Kumasaka, M., et al. (2007). Beta-catenin induces immortalization of melanocytes by suppressing p16INK4a expression and cooperates with N-Ras in melanoma development. Genes & Development, 21(22), 2923–2935. https://doi.org/10.1101/gad.450107.
Jiang, X., Hao, H.-X., Growney, J. D., Woolfenden, S., Bottiglio, C., Ng, N., et al. (2013). Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proceedings of the National Academy of Sciences of the United States of America, 110(31), 12649–12654. https://doi.org/10.1073/pnas.1307218110.
Zhong, Z., Sepramaniam, S., Chew, X. H., Wood, K., Lee, M. A., Madan, B., & Virshup, D. M. (2019). PORCN inhibition synergizes with PI3K/mTOR inhibition in Wnt-addicted cancers. Oncogene, 67(12), 7. https://doi.org/10.1038/s41388-019-0908-1.
Visvader, J. E., & Lindeman, G. J. (2008). Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature Reviews. Cancer, 8(10), 755–768. https://doi.org/10.1038/nrc2499.
Chang, J. C. (2016). Cancer stem cells: role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine, 95(1 Suppl 1), S20–S25. https://doi.org/10.1097/MD.0000000000004766.
Peitzsch, C., Tyutyunnykova, A., Pantel, K., & Dubrovska, A. (2017). Cancer stem cells: the root of tumor recurrence and metastases. Seminars in Cancer Biology, 44, 10–24. https://doi.org/10.1016/j.semcancer.2017.02.011.
Madan, B., Ke, Z., Harmston, N., Ho, S. Y., Frois, A. O., Alam, J., et al. (2016). Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene, 35(17), 2197–2207. https://doi.org/10.1038/onc.2015.280.
Steinhart, Z., Pavlovic, Z., Chandrashekhar, M., Hart, T., Wang, X., Zhang, X., et al. (2017). Genome-wide CRISPR screens reveal a Wnt-FZD5 signaling circuit as a druggable vulnerability of RNF43-mutant pancreatic tumors. Nature Medicine, 23(1), 60–68. https://doi.org/10.1038/nm.4219.
Milanovic, M., Fan, D. N. Y., Belenki, D., Däbritz, J. H. M., Zhao, Z., Yu, Y., et al. (2017). Senescence-associated reprogramming promotes cancer stemness. Nature, 15, 482. https://doi.org/10.1038/nature25167.
White, E., & DiPaola, R. S. (2009). The double-edged sword of autophagy modulation in cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 15(17), 5308–5316. https://doi.org/10.1158/1078-0432.CCR-07-5023.
Chen, S., Guttridge, D. C., You, Z., Zhang, Z., Fribley, A., Mayo, M. W., et al. (2001). Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. The Journal of Cell Biology, 152(1), 87–96. https://doi.org/10.1083/jcb.152.1.87.
Xie, H., Huang, Z., Sadim, M. S., & Sun, Z. (2005). Stabilized beta-catenin extends thymocyte survival by up-regulating Bcl-xL. The Journal of Immunology, 175(12), 7981–7988. https://doi.org/10.4049/jimmunol.175.12.7981.
Melkonyan, H. S., Chang, W. C., Shapiro, J. P., Mahadevappa, M., Fitzpatrick, P. A., Kiefer, M. C., et al. (1997). SARPs: a family of secreted apoptosis-related proteins. Proceedings of the National Academy of Sciences of the United States of America, 94(25), 13636–13641. https://doi.org/10.1073/pnas.94.25.13636.
Roth, W., Wild-Bode, C., Platten, M., Grimmel, C., Melkonyan, H. S., Dichgans, J., & Weller, M. (2000). Secreted frizzled-related proteins inhibit motility and promote growth of human malignant glioma cells. Oncogene, 19(37), 4210–4220. https://doi.org/10.1038/sj.onc.1203783.
Han, X., & Amar, S. (2004). Secreted frizzled-related protein 1 (SFRP1) protects fibroblasts from ceramide-induced apoptosis. The Journal of Biological Chemistry, 279(4), 2832–2840. https://doi.org/10.1074/jbc.M308102200.
Uren, A., Reichsman, F., Anest, V., Taylor, W. G., Muraiso, K., Bottaro, D. P., et al. (2000). Secreted frizzled-related protein-1 binds directly to wingless and is a biphasic modulator of Wnt signaling. The Journal of Biological Chemistry, 275(6), 4374–4382. https://doi.org/10.1074/jbc.275.6.4374.
Levine, B., & Kroemer, G. (2008). Autophagy in the pathogenesis of disease. Cell, 132(1), 27–42. https://doi.org/10.1016/j.cell.2007.12.018.
Sánchez-Martín, P., & Komatsu, M. (2018). p62/SQSTM1 - steering the cell through health and disease. Journal of Cell Science, 131(21), jcs222836. https://doi.org/10.1242/jcs.222836.
Lee, Y.-K., & Lee, J.-A. (2016). Role of the mammalian ATG8/LC3 family in autophagy: differential and compensatory roles in the spatiotemporal regulation of autophagy. BMB Reports, 49(8), 424–430. https://doi.org/10.5483/bmbrep.2016.49.8.081.
Gao, C., Cao, W., Bao, L., Zuo, W., Xie, G., Cai, T., et al. (2010). Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nature Cell Biology, 12(8), 781–790. https://doi.org/10.1038/ncb2082.
Petherick, K. J., Williams, A. C., Lane, J. D., Ordóñez-Morán, P., Huelsken, J., Collard, T. J., et al. (2013). Autolysosomal β-catenin degradation regulates Wnt-autophagy-p62 crosstalk. The EMBO Journal, 32(13), 1903–1916. https://doi.org/10.1038/emboj.2013.123.
Luo, X., Ye, S., Jiang, Q., Gong, Y., Yuan, Y., Hu, X., et al. (2018). Wnt inhibitory factor-1-mediated autophagy inhibits Wnt/β-catenin signaling by downregulating dishevelled-2 expression in non-small cell lung cancer cells. International Journal of Oncology, 53(2), 904–914. https://doi.org/10.3892/ijo.2018.4442.
Colella, B., Faienza, F., Carinci, M., D'Alessandro, G., Catalano, M., Santoro, A., et al. (2019). Autophagy induction impairs Wnt/β-catenin signalling through β-catenin relocalisation in glioblastoma cells. Cellular Signalling, 53, 357–364. https://doi.org/10.1016/j.cellsig.2018.10.017.
Nàger, M., Sallán, M. C., Visa, A., Pushparaj, C., Santacana, M., Macià, A., et al. (2018). Inhibition of WNT-CTNNB1 signaling upregulates SQSTM1 and sensitizes glioblastoma cells to autophagy blockers. Autophagy, 14(4), 619–636. https://doi.org/10.1080/15548627.2017.1423439.
Low, K. C., & Tergaonkar, V. (2013). Telomerase: central regulator of all of the hallmarks of cancer. Trends in Biochemical Sciences, 38(9), 426–434. https://doi.org/10.1016/j.tibs.2013.07.001.
Tang, R., Cheng, A. J., Wang, J. Y., & Wang, T. C. (1998). Close correlation between telomerase expression and adenomatous polyp progression in multistep colorectal carcinogenesis. Cancer Research, 58(18), 4052–4054.
Tahara, H., Kuniyasu, H., Yokozaki, H., Yasui, W., Shay, J. W., Ide, T., & Tahara, E. (1995). Telomerase activity in preneoplastic and neoplastic gastric and colorectal lesions. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 1(11), 1245–1251.
Mizumoto, I., Ogawa, Y., Niiyama, H., Nagai, E., Sato, I., Urashima, T., et al. (2001). Possible role of telomerase activation in the multistep tumor progression of periampullary lesions in patients with familial adenomatous polyposis. The American Journal of Gastroenterology, 96(4), 1261–1265. https://doi.org/10.1111/j.1572-0241.2001.03710.x.
Hoffmeyer, K., Raggioli, A., Rudloff, S., Anton, R., Hierholzer, A., Del Valle, I., et al. (2012). Wnt/β-catenin signaling regulates telomerase in stem cells and cancer cells. Science, 336(6088), 1549–1554. https://doi.org/10.1126/science.1218370.
Zhang, Y., Toh, L., Lau, P., & Wang, X. (2012). Human telomerase reverse transcriptase (hTERT) is a novel target of the Wnt/β-catenin pathway in human cancer. The Journal of Biological Chemistry, 287(39), 32494–32511. https://doi.org/10.1074/jbc.M112.368282.
Wang, J., Xie, L. Y., Allan, S., Beach, D., & Hannon, G. J. (1998). Myc activates telomerase. Genes & Development, 12(12), 1769–1774. https://doi.org/10.1101/gad.12.12.1769.
Strong, M. A., Vidal-Cardenas, S. L., Karim, B., Yu, H., Guo, N., & Greider, C. W. (2011). Phenotypes in mTERT+/− and mTERT−/− mice are due to short telomeres, not telomere-independent functions of telomerase reverse transcriptase. Molecular and Cellular Biology, 31(12), 2369–2379. https://doi.org/10.1128/MCB.05312-11.
Listerman, I., Gazzaniga, F. S., & Blackburn, E. H. (2014). An investigation of the effects of the core protein telomerase reverse transcriptase on Wnt signaling in breast cancer cells. Molecular and Cellular Biology, 34(2), 280–289. https://doi.org/10.1128/MCB.00844-13.
Yang, T.-L. B., Chen, Q., Deng, J. T., Jagannathan, G., Tobias, J. W., Schultz, D. C., et al. (2017). Mutual reinforcement between telomere capping and canonical Wnt signalling in the intestinal stem cell niche. Nature Communications, 8(1), 14766–14710. https://doi.org/10.1038/ncomms14766.
Wilson, C. L., Heppner, K. J., Labosky, P. A., Hogan, B. L., & Matrisian, L. M. (1997). Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proceedings of the National Academy of Sciences of the United States of America, 94(4), 1402–1407. https://doi.org/10.1073/pnas.94.4.1402.
Brabletz, T., Jung, A., Dag, S., Hlubek, F., & Kirchner, T. (1999). Beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. The American Journal of Pathology, 155(4), 1033–1038. https://doi.org/10.1016/s0002-9440(10)65204-2.
Lévy, L., Neuveut, C., Renard, C.-A., Charneau, P., Branchereau, S., Gauthier, F., et al. (2002). Transcriptional activation of interleukin-8 by beta-catenin-Tcf4. The Journal of Biological Chemistry, 277(44), 42386–42393. https://doi.org/10.1074/jbc.M207418200.
Li, A., Dubey, S., Varney, M. L., Dave, B. J., & Singh, R. K. (2003). IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. The Journal of Immunology, 170(6), 3369–3376. https://doi.org/10.4049/jimmunol.170.6.3369.
Ferrara, N., Hillan, K. J., Gerber, H.-P., & Novotny, W. (2004). Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nature Reviews Drug Discovery, 3(5), 391–400. https://doi.org/10.1038/nrd1381.
Krock, B. L., Skuli, N., & Simon, M. C. (2011). Hypoxia-induced angiogenesis: good and evil. Genes & Cancer, 2(12), 1117–1133. https://doi.org/10.1177/1947601911423654.
Zhang, X., Gaspard, J. P., & Chung, D. C. (2001). Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Research, 61(16), 6050–6054.
Easwaran, V., Lee, S. H., Inge, L., Guo, L., Goldbeck, C., Garrett, E., et al. (2003). Beta-catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Research, 63(12), 3145–3153.
Posokhova, E., Shukla, A., Seaman, S., Volate, S., Hilton, M. B., Wu, B., et al. (2015). GPR124 functions as a WNT7-specific coactivator of canonical β-catenin signaling. Cell Reports, 10(2), 123–130. https://doi.org/10.1016/j.celrep.2014.12.020.
Chang, J., Mancuso, M. R., Maier, C., Liang, X., Yuki, K., Yang, L., et al. (2017). Gpr124 is essential for blood-brain barrier integrity in central nervous system disease. Nature Medicine, 23(4), 450–460. https://doi.org/10.1038/nm.4309.
Cho, C., Smallwood, P. M., & Nathans, J. (2017). Reck and Gpr124 are essential receptor cofactors for Wnt7a/Wnt7b-specific signaling in mammalian CNS angiogenesis and blood-brain barrier regulation. Neuron, 95(5), 1056–1073.e5. https://doi.org/10.1016/j.neuron.2017.07.031.
Chaffer, C. L., & Weinberg, R. A. (2011). A perspective on cancer cell metastasis. Science, 331(6024), 1559–1564. https://doi.org/10.1126/science.1203543.
Fischer, K. R., Durrans, A., Lee, S., Sheng, J., Li, F., Wong, S. T. C., et al. (2015). Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature, 527(7579), 472–476. https://doi.org/10.1038/nature15748.
Zheng, X., Carstens, J. L., Kim, J., Scheible, M., Kaye, J., Sugimoto, H., et al. (2015). Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature, 527(7579), 525–530. https://doi.org/10.1038/nature16064.
Somarelli, J. A., Schaeffer, D., Marengo, M. S., Bepler, T., Rouse, D., Ware, K. E., et al. (2016). Distinct routes to metastasis: plasticity-dependent and plasticity-independent pathways. Oncogene. https://doi.org/10.1038/onc.2015.497.
Lambert, A. W., Pattabiraman, D. R., & Weinberg, R. A. (2017). Emerging biological principles of metastasis. Cell, 168(4), 670–691. https://doi.org/10.1016/j.cell.2016.11.037.
Brabletz, T., Jung, A., Hermann, K., Günther, K., Hohenberger, W., & Kirchner, T. (1998). Nuclear overexpression of the oncoprotein beta-catenin in colorectal cancer is localized predominantly at the invasion front. Pathology, Research and Practice, 194(10), 701–704. https://doi.org/10.1016/s0344-0338(98)80129-5.
Brabletz, T., Jung, A., Reu, S., Porzner, M., Hlubek, F., Kunz-Schughart, L. A., et al. (2001). Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proceedings of the National Academy of Sciences of the United States of America, 98(18), 10356–10361. https://doi.org/10.1073/pnas.171610498.
Vermeulen, L., de Sousa e Melo, F., van der Heijden, M., Cameron, K., de Jong, J. H., Borovski, T., et al. (2010). Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature Cell Biology, 12(5), 468–476. https://doi.org/10.1038/ncb2048.
Maruyama, K., Ochiai, A., Akimoto, S., Nakamura, S., Baba, S., Moriya, Y., & Hirohashi, S. (2000). Cytoplasmic beta-catenin accumulation as a predictor of hematogenous metastasis in human colorectal cancer. Oncology, 59(4), 302–309. https://doi.org/10.1159/000012187.
Hörkkö, T. T., Klintrup, K., Mäkinen, J. M., Näpänkangas, J. B., Tuominen, H. J., Mäkelä, J., et al. (2006). Budding invasive margin and prognosis in colorectal cancer--no direct association with beta-catenin expression. European Journal of Cancer, 42(7), 964–971. https://doi.org/10.1016/j.ejca.2006.01.017.
Horst, D., Reu, S., Kriegl, L., Engel, J., Kirchner, T., & Jung, A. (2009). The intratumoral distribution of nuclear beta-catenin is a prognostic marker in colon cancer. Cancer, 115(10), 2063–2070. https://doi.org/10.1002/cncr.24254.
Gao, Z.-H., Lu, C., Wang, M.-X., Han, Y., & Guo, L.-J. (2014). Differential β-catenin expression levels are associated with morphological features and prognosis of colorectal cancer. Oncology Letters, 8(5), 2069–2076. https://doi.org/10.3892/ol.2014.2433.
Liu, L., Zhu, X.-D., Wang, W.-Q., Shen, Y., Qin, Y., Ren, Z.-G., et al. (2010). Activation of beta-catenin by hypoxia in hepatocellular carcinoma contributes to enhanced metastatic potential and poor prognosis. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 16(10), 2740–2750. https://doi.org/10.1158/1078-0432.CCR-09-2610.
Yook, J. I., Li, X.-Y., Ota, I., Fearon, E. R., & Weiss, S. J. (2005). Wnt-dependent regulation of the E-cadherin repressor snail. The Journal of Biological Chemistry, 280(12), 11740–11748. https://doi.org/10.1074/jbc.M413878200.
Esposito, M., Mondal, N., Greco, T. M., Wei, Y., Spadazzi, C., Lin, S.-C., et al. (2019). Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nature Cell Biology, 21(5), 627–639. https://doi.org/10.1038/s41556-019-0309-2.
Nandana, S., Tripathi, M., Duan, P., Chu, C.-Y., Mishra, R., Liu, C., et al. (2017). Bone metastasis of prostate cancer can be therapeutically targeted at the TBX2-WNT signaling axis. Cancer Research, 77(6), 1331–1344. https://doi.org/10.1158/0008-5472.CAN-16-0497.
Nguyen, D. X., Chiang, A. C., Zhang, X. H.-F., Kim, J. Y., Kris, M. G., Ladanyi, M., et al. (2009). WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell, 138(1), 51–62. https://doi.org/10.1016/j.cell.2009.04.030.
Han, F., Liu, W.-B., Shi, X.-Y., Yang, J.-T., Zhang, X., Li, Z.-M., et al. (2018). SOX30 inhibits tumor metastasis through attenuating Wnt-signaling via transcriptional and posttranslational regulation of β-catenin in lung cancer. EBioMedicine, 31, 253–266. https://doi.org/10.1016/j.ebiom.2018.04.026.
Cai, J., Fang, L., Huang, Y., Li, R., Xu, X., Hu, Z., et al. (2017). Simultaneous overactivation of Wnt/β-catenin and TGFβ signalling by miR-128-3p confers chemoresistance-associated metastasis in NSCLC. Nature Communications, 8(1), 15870–15819. https://doi.org/10.1038/ncomms15870.
Dai, F.-Q., Li, C.-R., Fan, X.-Q., Tan, L., Wang, R.-T., & Jin, H. (2019). miR-150-5p inhibits non-small-cell lung cancer metastasis and recurrence by targeting HMGA2 and β-catenin signaling. Molecular Therapy--Nucleic Acids, 16, 675–685. https://doi.org/10.1016/j.omtn.2019.04.017.
Wellenstein, M. D., Coffelt, S. B., Duits, D. E. M., van Miltenburg, M. H., Slagter, M., de Rink, I., et al. (2019). Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature, 15, e493. https://doi.org/10.1038/s41586-019-1450-6.
Tenbaum, S. P., Ordóñez-Morán, P., Puig, I., Chicote, I., Arqués, O., Landolfi, S., et al. (2012). β-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nature Medicine, 18(6), 892–901. https://doi.org/10.1038/nm.2772.
Luga, V., Zhang, L., Viloria-Petit, A. M., Ogunjimi, A. A., Inanlou, M. R., Chiu, E., et al. (2012). Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell, 151(7), 1542–1556. https://doi.org/10.1016/j.cell.2012.11.024.
Yu, M., Ting, D. T., Stott, S. L., Wittner, B. S., Ozsolak, F., Paul, S., et al. (2012). RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature, 487(7408), 510–513. https://doi.org/10.1038/nature11217.
Miyamoto, D. T., Zheng, Y., Wittner, B. S., Lee, R. J., Zhu, H., Broderick, K. T., et al. (2015). RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science, 349(6254), 1351–1356. https://doi.org/10.1126/science.aab0917.
Li, F., Tiede, B., Massagué, J., & Kang, Y. (2007). Beyond tumorigenesis: cancer stem cells in metastasis. Cell Research, 17(1), 3–14. https://doi.org/10.1038/sj.cr.7310118.
Shiozawa, Y., Nie, B., Pienta, K. J., Morgan, T. M., & Taichman, R. S. (2013). Cancer stem cells and their role in metastasis. Pharmacology & Therapeutics, 138(2), 285–293. https://doi.org/10.1016/j.pharmthera.2013.01.014.
Alexandrov, L. B., Kim, J., Haradhvala, N. J., Huang, M. N., Tian Ng, A. W., Wu, Y., et al. (2020). The repertoire of mutational signatures in human cancer. Nature, 578(7793), 94–101. https://doi.org/10.1038/s41586-020-1943-3.
Rusan, N. M., & Peifer, M. (2008). Original CIN: reviewing roles for APC in chromosome instability. The Journal of Cell Biology, 181(5), 719–726. https://doi.org/10.1083/jcb.200802107.
Näthke, I. S. (2004). The adenomatous polyposis coli protein: the Achilles heel of the gut epithelium. Annual Review of Cell and Developmental Biology, 20(1), 337–366. https://doi.org/10.1146/annurev.cellbio.20.012103.094541.
Munemitsu, S., Souza, B., Müller, O., Albert, I., Rubinfeld, B., & Polakis, P. (1994). The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Research, 54(14), 3676–3681.
Smith, K. J., Levy, D. B., Maupin, P., Pollard, T. D., Vogelstein, B., & Kinzler, K. W. (1994). Wild-type but not mutant APC associates with the microtubule cytoskeleton. Cancer Research, 54(14), 3672–3675.
Zumbrunn, J., Kinoshita, K., Hyman, A. A., & Näthke, I. S. (2001). Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Current Biology: CB, 11(1), 44–49. https://doi.org/10.1016/s0960-9822(01)00002-1.
Fodde, R., Kuipers, J., Rosenberg, C., Smits, R., Kielman, M., Gaspar, C., et al. (2001). Mutations in the APC tumour suppressor gene cause chromosomal instability. Nature Cell Biology, 3(4), 433–438. https://doi.org/10.1038/35070129.
Banks, J. D., & Heald, R. (2004). Adenomatous polyposis coli associates with the microtubule-destabilizing protein XMCAK. Current Biology: CB, 14(22), 2033–2038. https://doi.org/10.1016/j.cub.2004.10.049.
Tighe, A., Johnson, V. L., & Taylor, S. S. (2004). Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability. Journal of Cell Science, 117(Pt 26), 6339–6353. https://doi.org/10.1242/jcs.01556.
Draviam, V. M., Shapiro, I., Aldridge, B., & Sorger, P. K. (2006). Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1- or APC-depleted cells. The EMBO Journal, 25(12), 2814–2827. https://doi.org/10.1038/sj.emboj.7601168.
Hadjihannas, M. V., Brückner, M., Jerchow, B., Birchmeier, W., Dietmaier, W., & Behrens, J. (2006). Aberrant Wnt/beta-catenin signaling can induce chromosomal instability in colon cancer. Proceedings of the National Academy of Sciences of the United States of America, 103(28), 10747–10752. https://doi.org/10.1073/pnas.0604206103.
Hadjihannas, M. V., Brückner, M., & Behrens, J. (2010). Conductin/axin2 and Wnt signalling regulates centrosome cohesion. EMBO Reports, 11(4), 317–324. https://doi.org/10.1038/embor.2010.23.
Fumoto, K., Kadono, M., Izumi, N., & Kikuchi, A. (2009). Axin localizes to the centrosome and is involved in microtubule nucleation. EMBO Reports, 10(6), 606–613. https://doi.org/10.1038/embor.2009.45.
Caldwell, C. M., Green, R. A., & Kaplan, K. B. (2007). APC mutations lead to cytokinetic failures in vitro and tetraploid genotypes in Min mice. The Journal of Cell Biology, 178(7), 1109–1120. https://doi.org/10.1083/jcb.200703186.
Aoki, K., Aoki, M., Sugai, M., Harada, N., Miyoshi, H., Tsukamoto, T., et al. (2007). Chromosomal instability by beta-catenin/TCF transcription in APC or beta-catenin mutant cells. Oncogene, 26(24), 3511–3520. https://doi.org/10.1038/sj.onc.1210141.
Voloshanenko, O., Erdmann, G., Dubash, T. D., Augustin, I., Metzig, M., Moffa, G., et al. (2013). Wnt secretion is required to maintain high levels of Wnt activity in colon cancer cells. Nature Communications, 4(1), 2610. https://doi.org/10.1038/ncomms3610.
Srinivas, U. S., Tan, B. W. Q., Vellayappan, B. A., & Jeyasekharan, A. D. (2019). ROS and the DNA damage response in cancer. Redox Biology, 25, 101084. https://doi.org/10.1016/j.redox.2018.101084.
Tichy, E. D., Pillai, R., Deng, L., Tischfield, J. A., Hexley, P., Babcock, G. F., & Stambrook, P. J. (2012). The abundance of Rad51 protein in mouse embryonic stem cells is regulated at multiple levels. Stem Cell Research, 9(2), 124–134. https://doi.org/10.1016/j.scr.2012.05.004.
Bao, S., Wu, Q., McLendon, R. E., Hao, Y., Shi, Q., Hjelmeland, A. B., et al. (2006). Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature, 444(7120), 756–760. https://doi.org/10.1038/nature05236.
Gibson, B. A., & Kraus, W. L. (2012). New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nature Reviews. Molecular Cell Biology, 13(7), 411–424. https://doi.org/10.1038/nrm3376.
Eustermann, S., Videler, H., Yang, J.-C., Cole, P. T., Gruszka, D., Veprintsev, D., & Neuhaus, D. (2011). The DNA-binding domain of human PARP-1 interacts with DNA single-strand breaks as a monomer through its second zinc finger. Journal of Molecular Biology, 407(1), 149–170. https://doi.org/10.1016/j.jmb.2011.01.034.
Armstrong, A. C., & Clay, V. (2019). Olaparib in germline-mutated metastatic breast cancer: implications of the OlympiAD trial. Future Oncology (London, England), 15(20), 2327–2335. https://doi.org/10.2217/fon-2018-0067.
Gelmon, K. A., Tischkowitz, M., Mackay, H., Swenerton, K., Robidoux, A., Tonkin, K., et al. (2011). Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. The Lancet Oncology, 12(9), 852–861. https://doi.org/10.1016/S1470-2045(11)70214-5.
Yamamoto, T. M., McMellen, A., Watson, Z. L., Aguilera, J., Ferguson, R., Nurmemmedov, E., et al. (2019). Activation of Wnt signaling promotes olaparib resistant ovarian cancer. Molecular Carcinogenesis, 68, 7. https://doi.org/10.1002/mc.23064.
Thorne, C. A., Hanson, A. J., Schneider, J., Tahinci, E., Orton, D., Cselenyi, C. S., et al. (2010). Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α. Nature Chemical Biology, 6(11), 829–836. https://doi.org/10.1038/nchembio.453.
Li, B., Fei, D. L., Flaveny, C. A., Dahmane, N., Baubet, V., Wang, Z., et al. (2014). Pyrvinium attenuates Hedgehog signaling downstream of smoothened. Cancer Research, 74(17), 4811–4821. https://doi.org/10.1158/0008-5472.CAN-14-0317.
Jun, S., Jung, Y.-S., Suh, H. N., Wang, W., Kim, M. J., Oh, Y. S., et al. (2016). LIG4 mediates Wnt signalling-induced radioresistance. Nature Communications, 7(1), 10994–10913. https://doi.org/10.1038/ncomms10994.
Kanazawa, A., Tsukada, S., Sekine, A., Tsunoda, T., Takahashi, A., Kashiwagi, A., et al. (2004). Association of the gene encoding wingless-type mammary tumor virus integration-site family member 5B (WNT5B) with type 2 diabetes. American Journal of Human Genetics, 75(5), 832–843. https://doi.org/10.1086/425340.
Zeve, D., Seo, J., Suh, J. M., Stenesen, D., Tang, W., Berglund, E. D., et al. (2012). Wnt signaling activation in adipose progenitors promotes insulin-independent muscle glucose uptake. Cell Metabolism, 15(4), 492–504. https://doi.org/10.1016/j.cmet.2012.03.010.
Mori, H., Prestwich, T. C., Reid, M. A., Longo, K. A., Gerin, I., Cawthorn, W. P., et al. (2012). Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. The Journal of Clinical Investigation, 122(7), 2405–2416. https://doi.org/10.1172/JCI63604.
Behari, J., Li, H., Liu, S., Stefanovic-Racic, M., Alonso, L., O'Donnell, C. P., et al. (2014). β-catenin links hepatic metabolic zonation with lipid metabolism and diet-induced obesity in mice. The American Journal of Pathology, 184(12), 3284–3298. https://doi.org/10.1016/j.ajpath.2014.08.022.
Sethi, J. K., & Vidal-Puig, A. (2010). Wnt signalling and the control of cellular metabolism. The Biochemical Journal, 427(1), 1–17. https://doi.org/10.1042/BJ20091866.
Liberti, M. V., & Locasale, J. W. (2016). The Warburg effect: how does it benefit cancer cells? Trends in Biochemical Sciences, 41(3), 211–218. https://doi.org/10.1016/j.tibs.2015.12.001.
Sherwood, V. (2015). WNT signaling: an emerging mediator of cancer cell metabolism? Molecular and Cellular Biology, 35(1), 2–10. https://doi.org/10.1128/MCB.00992-14.
Chafey, P., Finzi, L., Boisgard, R., Caüzac, M., Clary, G., Broussard, C., et al. (2009). Proteomic analysis of beta-catenin activation in mouse liver by DIGE analysis identifies glucose metabolism as a new target of the Wnt pathway. Proteomics, 9(15), 3889–3900. https://doi.org/10.1002/pmic.200800609.
Pate, K. T., Stringari, C., Sprowl-Tanio, S., Wang, K., TeSlaa, T., Hoverter, N. P., et al. (2014). Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. The EMBO Journal, 33(13), 1454–1473. https://doi.org/10.15252/embj.201488598.
Lee, S. Y., Jeon, H. M., Ju, M. K., Kim, C. H., Yoon, G., Han, S. I., et al. (2012). Wnt/Snail signaling regulates cytochrome C oxidase and glucose metabolism. Cancer Research, 72(14), 3607–3617. https://doi.org/10.1158/0008-5472.CAN-12-0006.
Dang, C. V., Le, A., & Gao, P. (2009). MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 15(21), 6479–6483. https://doi.org/10.1158/1078-0432.CCR-09-0889.
Coussens, L. M., & Werb, Z. (2002). Inflammation and cancer. Nature, 420(6917), 860–867. https://doi.org/10.1038/nature01322.
Crusz, S. M., & Balkwill, F. R. (2015). Inflammation and cancer: advances and new agents. Nature Reviews. Clinical Oncology, 12(10), 584–596. https://doi.org/10.1038/nrclinonc.2015.105.
Yeo, E.-J., Cassetta, L., Qian, B.-Z., Lewkowich, I., Li, J.-F., Stefater, J. A., et al. (2014). Myeloid WNT7b mediates the angiogenic switch and metastasis in breast cancer. Cancer Research, 74(11), 2962–2973. https://doi.org/10.1158/0008-5472.CAN-13-2421.
Ojalvo, L. S., Whittaker, C. A., Condeelis, J. S., & Pollard, J. W. (2010). Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. The Journal of Immunology, 184(2), 702–712. https://doi.org/10.4049/jimmunol.0902360.
Oguma, K., Oshima, H., Aoki, M., Uchio, R., Naka, K., Nakamura, S., et al. (2008). Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. The EMBO Journal, 27(12), 1671–1681. https://doi.org/10.1038/emboj.2008.105.
Pukrop, T., Klemm, F., Hagemann, T., Gradl, D., Schulz, M., Siemes, S., et al. (2006). Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proceedings of the National Academy of Sciences of the United States of America, 103(14), 5454–5459. https://doi.org/10.1073/pnas.0509703103.
Linde, N., Casanova-Acebes, M., Sosa, M. S., Mortha, A., Rahman, A., Farias, E., et al. (2018). Macrophages orchestrate breast cancer early dissemination and metastasis. Nature Communications, 9(1), 21. https://doi.org/10.1038/s41467-017-02481-5.
Manicassamy, S., Reizis, B., Ravindran, R., Nakaya, H., Salazar-Gonzalez, R. M., Wang, Y.-C., & Pulendran, B. (2010). Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science, 329(5993), 849–853. https://doi.org/10.1126/science.1188510.
Oderup, C., LaJevic, M., & Butcher, E. C. (2013). Canonical and noncanonical Wnt proteins program dendritic cell responses for tolerance. The Journal of Immunology, 190(12), 6126–6134. https://doi.org/10.4049/jimmunol.1203002.
Hong, Y., Manoharan, I., Suryawanshi, A., Shanmugam, A., Swafford, D., Ahmad, S., et al. (2016). Deletion of LRP5 and LRP6 in dendritic cells enhances antitumor immunity. Oncoimmunology, 5(4), e1115941. https://doi.org/10.1080/2162402X.2015.1115941.
Spranger, S., Bao, R., & Gajewski, T. F. (2015). Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature, 523(7559), 231–235. https://doi.org/10.1038/nature14404.
Luke, J. J., Bao, R., Sweis, R. F., Spranger, S., & Gajewski, T. F. (2019). WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, clincanres.1942.2018. https://doi.org/10.1158/1078-0432.CCR-18-1942.
Holtzhausen, A., Zhao, F., Evans, K. S., Tsutsui, M., Orabona, C., Tyler, D. S., & Hanks, B. A. (2015). Melanoma-derived Wnt5a promotes local dendritic-cell expression of IDO and immunotolerance: opportunities for pharmacologic enhancement of immunotherapy. Cancer Immunology Research, 3(9), 1082–1095. https://doi.org/10.1158/2326-6066.CIR-14-0167.
Fallarino, F., Grohmann, U., You, S., McGrath, B. C., Cavener, D. R., Vacca, C., et al. (2006). The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. The Journal of Immunology, 176(11), 6752–6761. https://doi.org/10.4049/jimmunol.176.11.6752.
Zhao, F., Xiao, C., Evans, K. S., Theivanthiran, T., DeVito, N., Holtzhausen, A., et al. (2018). Paracrine Wnt5a-β-catenin signaling triggers a metabolic program that drives dendritic cell tolerization. Immunity, 48(1), 147–160.e7. https://doi.org/10.1016/j.immuni.2017.12.004.
Khazaie, K., Blatner, N. R., Khan, M. W., Gounari, F., Gounaris, E., Dennis, K., et al. (2011). The significant role of mast cells in cancer. Cancer Metastasis Reviews, 30(1), 45–60. https://doi.org/10.1007/s10555-011-9286-z.
Yamaguchi, T., Nishijima, M., Tashiro, K., & Kawabata, K. (2016). Wnt-β-catenin signaling promotes the maturation of mast cells. BioMed Research International, 2016(7, article 1378), 2048987–2048988. https://doi.org/10.1155/2016/2048987.
Tebroke, J., Lieverse, J. E., Säfholm, J., Schulte, G., Nilsson, G., & Rönnberg, E. (2019). Wnt-3a induces cytokine release in human mast cells. Cells, 8(11), 1372. https://doi.org/10.3390/cells8111372.
Strouch, M. J., Cheon, E. C., Salabat, M. R., Krantz, S. B., Gounaris, E., Melstrom, L. G., et al. (2010). Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 16(8), 2257–2265. https://doi.org/10.1158/1078-0432.CCR-09-1230.
Jensen, H. K., Donskov, F., Marcussen, N., Nordsmark, M., Lundbeck, F., & von der Maase, H. (2009). Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 27(28), 4709–4717. https://doi.org/10.1200/JCO.2008.18.9498.
Trellakis, S., Bruderek, K., Dumitru, C. A., Gholaman, H., Gu, X., Bankfalvi, A., et al. (2011). Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. International Journal of Cancer, 129(9), 2183–2193. https://doi.org/10.1002/ijc.25892.
Galdiero, M. R., Bianchi, P., Grizzi, F., Di Caro, G., Basso, G., Ponzetta, A., et al. (2016). Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. International Journal of Cancer, 139(2), 446–456. https://doi.org/10.1002/ijc.30076.
Caruso, R. A., Bellocco, R., Pagano, M., Bertoli, G., Rigoli, L., & Inferrera, C. (2002). Prognostic value of intratumoral neutrophils in advanced gastric carcinoma in a high-risk area in northern Italy. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc, 15(8), 831–837. https://doi.org/10.1097/01.MP.0000020391.98998.6B.
Chao, T., Furth, E. E., & Vonderheide, R. H. (2016). CXCR2-dependent accumulation of tumor-associated neutrophils regulates T-cell immunity in pancreatic ductal adenocarcinoma. Cancer Immunology Research, 4(11), 968–982. https://doi.org/10.1158/2326-6066.CIR-16-0188.
Skokowa, J., Cario, G., Uenalan, M., Schambach, A., Germeshausen, M., Battmer, K., et al. (2006). LEF-1 is crucial for neutrophil granulocytopoiesis and its expression is severely reduced in congenital neutropenia. Nature Medicine, 12(10), 1191–1197. https://doi.org/10.1038/nm1474.
Verbeek, S., Izon, D., Hofhuis, F., Robanus-Maandag, E., te Riele, H., van de Wetering, M., et al. (1995). An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature, 374(6517), 70–74. https://doi.org/10.1038/374070a0.
Staal, F. J., Meeldijk, J., Moerer, P., Jay, P., van de Weerdt, B. C., Vainio, S., et al. (2001). Wnt signaling is required for thymocyte development and activates Tcf-1 mediated transcription. European Journal of Immunology, 31(1), 285–293. https://doi.org/10.1002/1521-4141(200101)31:1<285::AID-IMMU285>3.0.CO;2-D.
Wang, B., Tian, T., Kalland, K.-H., Ke, X., & Qu, Y. (2018). Targeting Wnt/β-catenin signaling for cancer immunotherapy. Trends in Pharmacological Sciences, 39(7), 648–658. https://doi.org/10.1016/j.tips.2018.03.008.
Wong, C., Chen, C., Wu, Q., Liu, Y., & Zheng, P. (2015). A critical role for the regulated wnt-myc pathway in naive T cell survival. The Journal of Immunology, 194(1), 158–167. https://doi.org/10.4049/jimmunol.1401238.
Yu, Q., Sharma, A., Oh, S. Y., Moon, H.-G., Hossain, M. Z., Salay, T. M., et al. (2009). T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nature Immunology, 10(9), 992–999. https://doi.org/10.1038/ni.1762.
Notani, D., Gottimukkala, K. P., Jayani, R. S., Limaye, A. S., Damle, M. V., Mehta, S., et al. (2010). Global regulator SATB1 recruits beta-catenin and regulates T(H)2 differentiation in Wnt-dependent manner. PLoS Biology, 8(1), e1000296. https://doi.org/10.1371/journal.pbio.1000296.
Dai, W., Liu, F., Li, C., Lu, Y., Lu, X., Du, S., et al. (2016). Blockade of Wnt/β-catenin pathway aggravated silica-induced lung inflammation through Tregs regulation on Th immune responses. Mediators of Inflammation, 2016(6), 6235614–6235614. https://doi.org/10.1155/2016/6235614.
Ding, Y., Shen, S., Lino, A. C., Curotto de Lafaille, M. A., & Lafaille, J. J. (2008). Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nature Medicine, 14(2), 162–169. https://doi.org/10.1038/nm1707.
Sun, X., Liu, S., Wang, D., Zhang, Y., Li, W., Guo, Y., et al. (2017). Colorectal cancer cells suppress CD4+ T cells immunity through canonical Wnt signaling. Oncotarget, 8(9), 15168–15181. https://doi.org/10.18632/oncotarget.14834.
Keerthivasan, S., Aghajani, K., Dose, M., Molinero, L., Khan, M. W., Venkateswaran, V., et al. (2014). β-Catenin promotes colitis and colon cancer through imprinting of proinflammatory properties in T cells. Science Translational Medicine, 6(225), 225ra28–225ra28. https://doi.org/10.1126/scitranslmed.3007607.
Trujillo, J. A., Sweis, R. F., Bao, R., & Luke, J. J. (2018). T cell-inflamed versus non-T cell-inflamed tumors: a conceptual framework for cancer immunotherapy drug development and combination therapy selection. Cancer Immunology Research, 6(9), 990–1000. https://doi.org/10.1158/2326-6066.CIR-18-0277.
Spranger, S., & Gajewski, T. F. (2018). Impact of oncogenic pathways on evasion of antitumour immune responses. Nature Reviews. Cancer, 18(3), 139–147. https://doi.org/10.1038/nrc.2017.117.
Sweis, R. F., Spranger, S., Bao, R., Paner, G. P., Stadler, W. M., Steinberg, G., & Gajewski, T. F. (2016). Molecular drivers of the non-T-cell-inflamed tumor microenvironment in urothelial bladder cancer. Cancer Immunology Research, 4(7), 563–568. https://doi.org/10.1158/2326-6066.CIR-15-0274.
Murata, Y., Saito, Y., Kotani, T., & Matozaki, T. (2018). CD47-signal regulatory protein α signaling system and its application to cancer immunotherapy. Cancer Science, 109(8), 2349–2357. https://doi.org/10.1111/cas.13663.
Gowda, P., Patrick, S., Singh, A., Sheikh, T., & Sen, E. (2018). Mutant isocitrate dehydrogenase 1 disrupts PKM2-β-catenin-BRG1 transcriptional network-driven CD47 expression. Molecular and Cellular Biology, 38(9), 597. https://doi.org/10.1128/MCB.00001-18.
Buchbinder, E. I., & Desai, A. (2016). CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. American Journal of Clinical Oncology, 39(1), 98–106. https://doi.org/10.1097/COC.0000000000000239.
Hui, E., Cheung, J., Zhu, J., Su, X., Taylor, M. J., Wallweber, H. A., et al. (2017). T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science, 355(6332), 1428–1433. https://doi.org/10.1126/science.aaf1292.
Yokosuka, T., Takamatsu, M., Kobayashi-Imanishi, W., Hashimoto-Tane, A., Azuma, M., & Saito, T. (2012). Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. The Journal of Experimental Medicine, 209(6), 1201–1217. https://doi.org/10.1084/jem.20112741.
Chovanec, M., Cierna, Z., Miskovska, V., Machalekova, K., Kalavska, K., Rejlekova, K., et al. (2018). βcatenin is a marker of poor clinical characteristics and suppressed immune infiltration in testicular germ cell tumors. BMC Cancer, 18(1), 1062–1010. https://doi.org/10.1186/s12885-018-4929-x.
Castagnoli, L., Cancila, V., Cordoba-Romero, S. L., Faraci, S., Talarico, G., Belmonte, B., et al. (2019). WNT signaling modulates PD-L1 expression in the stem cell compartment of triple-negative breast cancer. Oncogene, 38(21), 4047–4060. https://doi.org/10.1038/s41388-019-0700-2.
Casey, S. C., Tong, L., Li, Y., Do, R., Walz, S., Fitzgerald, K. N., et al. (2016). MYC regulates the antitumor immune response through CD47 and PD-L1. Science, 352(6282), 227–231. https://doi.org/10.1126/science.aac9935.
Kalbasi, A., & Ribas, A. (2020). Tumour-intrinsic resistance to immune checkpoint blockade. Nature Reviews. Immunology, 20(1), 25–39. https://doi.org/10.1038/s41577-019-0218-4.
Rotte, A. (2019). Combination of CTLA-4 and PD-1 blockers for treatment of cancer. Journal of Experimental and Clinical Cancer Research, 38(1), 255–212. https://doi.org/10.1186/s13046-019-1259-z.
Shah, K. V., Chien, A. J., Yee, C., & Moon, R. T. (2008). CTLA-4 is a direct target of Wnt/beta-catenin signaling and is expressed in human melanoma tumors. The Journal of Investigative Dermatology, 128(12), 2870–2879. https://doi.org/10.1038/jid.2008.170.
Blando, J., Sharma, A., Higa, M. G., Zhao, H., Vence, L., Yadav, S. S., et al. (2019). Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America, 116(5), 1692–1697. https://doi.org/10.1073/pnas.1811067116.
Guo, Y., Xiao, L., Sun, L., & Liu, F. (2012). Wnt/beta-catenin signaling: a promising new target for fibrosis diseases. Physiological Research, 61(4), 337–346.
Madan, B., Patel, M. B., Zhang, J., Bunte, R. M., Rudemiller, N. P., Griffiths, R., et al. (2016). Experimental inhibition of porcupine-mediated Wnt O-acylation attenuates kidney fibrosis. Kidney International. https://doi.org/10.1016/j.kint.2016.01.017.
Madan, B., & Virshup, D. M. (2015). Targeting Wnts at the source--new mechanisms, new biomarkers, new drugs. Molecular Cancer Therapeutics, 14(5), 1087–1094. https://doi.org/10.1158/1535-7163.MCT-14-1038.
Acknowledgments
We gratefully acknowledge the assistance of Nakeisha Tan for the illustrations.
Funding
The authors received support from the Singapore Ministry of Health’s National Medical Research Council Open Fund–Independent Research grant NMRC/OFIRG/0055/2017 (to Babita Madan) and the STAR Award Program MOH-000155 (to David Virshup).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhong, Z., Yu, J., Virshup, D.M. et al. Wnts and the hallmarks of cancer. Cancer Metastasis Rev 39, 625–645 (2020). https://doi.org/10.1007/s10555-020-09887-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-020-09887-6