Abstract
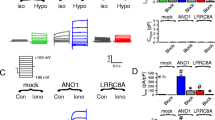
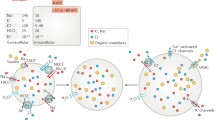
During cell swelling, Cl− channels are activated to lower intracellular Cl− concentrations and to reduce cell volume, a process termed regulatory volume decrease (RVD). We show that anoctamin 6 (ANO6; TMEM16F) produces volume-regulated anion currents and controls cell volume in four unrelated cell types. Volume regulation is compromised in freshly isolated intestinal epithelial cells from Ano6−/− mice and also in lymphocytes from a patient lacking expression of ANO6. Ca2+ influx is activated and thus ANO6 is stimulated during cell swelling by local Ca2+ increase probably in functional nanodomains near the plasma membrane. This leads to stimulation of phospholipase A2 (PLA2) and generation of plasma membrane lysophospholipids, which activates ANO6. Direct application of lysophospholipids also activates an anion current that is inhibited by typical ANO6 blocker. An increase in intracellular Ca2+ supports activation of ANO6, but is not required when PLA2 is fully activated, while re-addition of arachidonic acid completely blocked ANO6. Moreover, ANO6 is activated by low intracellular Cl− concentrations and may therefore operate as a cellular osmosensor. High intracellular Cl− concentration inhibits ANO6 and activation by PLA2. Taken together, ANO6 supports volume regulation and volume activation of anion currents by action as a Cl− channel or by scrambling membrane phospholipids. Thereby, it may support the function of LRRC8 proteins.






Similar content being viewed by others
References
Akita T, Okada Y (2011) Regulation of bradykinin-induced activation of volume-sensitive outwardly rectifying anion channels by Ca2+ nanodomains in mouse astrocytes. J Physiol 589:3909–3927
Almaca J, Tian Y, AlDehni F, Ousingsawat J, Kongsuphol P, Rock JR, Harfe BD, Schreiber R, Kunzelmann K (2009) TMEM16 proteins produce volume regulated chloride currents that are reduced in mice lacking TMEM16A. J Biol Chem 284:28571–28578
Berridge MJ (2004) Conformational coupling: a physiological calcium entry mechanism. Sci STKE 2004:e33
Bessac BF, Fleig A (2007) TRPM7 channel is sensitive to osmotic gradients in human kidney cells. J Physiol 582:1073–1086
Brunner JD, Lim NK, Schenck S, Duerst A, Dutzler R (2014) X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature 516:207–212
Cruz-Rangel S, Gamba G, Ramos-Mandujano G, Pasantes-Morales H (2012) Influence of WNK3 on intracellular chloride concentration and volume regulation in HEK293 cells. Pflugers Arch 464:317–330
Dawson DC, Van Driessche W, Helman SI (1988) Osmotically induced basolateral K+ conductance in turtle colon: lidocaine-induced K+ channel noise. Am J Physiol 254:C165–C174
Ehlen HW, Chinenkova M, Moser M, Munter HM, Krause Y, Gross S, Brachvogel B, Wuelling M, Kornak U, Vortkamp A (2012) Inactivation of Anoctamin-6/Tmem16f, a regulator of phosphatidylserine scrambling in osteoblasts, leads to decreased mineral deposition in skeletal tissues. J Bone Miner Res 28:246–259
Fischer KG, Leipziger J, Rubini-Illes P, Nitschke R, Greger R (1996) Attenuation of stimulated Ca2+ influx in colonic epithelial (HT29) cells by cAMP. Pflugers Arch 432:735–740
Forrest LR, Tavoulari S, Zhang YW, Rudnick G, Honig B (2007) Identification of a chloride ion binding site in Na+/Cl-dependent transporters. Proc Natl Acad Sci U S A 104:12761–12766
Grubb S, Poulsen KA, Juul CA, Kyed T, Klausen TK, Larsen EH, Hoffmann EK (2013) TMEM16F (anoctamin 6), an anion channel of delayed Ca2+ activation. J Gen Physiol 141:585–600
Harteneck C, Frenzel H, Kraft R (2007) N-(p-amylcinnamoyl)anthranilic acid (ACA): a phospholipase A(2) inhibitor and TRP channel blocker. Cardiovasc Drug Rev 25:61–75
Harteneck C, Gollasch M (2011) Pharmacological modulation of diacylglycerol-sensitive TRPC3/6/7 channels. Curr Pharm Biotechnol 12:35–41
Helix N, Strobaek D, Dahl BH, Christophersen P (2003) Inhibition of the endogenous volume-regulated anion channel (VRAC) in HEK293 cells by acidic di-aryl-ureas. J Membr Biol 196:83–94
Hoffmann EK, Lambert IH, Pedersen SF (2009) Physiology of cell volume regulation in vertebrates. Physiol Rev 89:193–277
Ishii T, Hashimoto T, Ohmori H (1996) Hypotonic stimulation induced Ca2+ release from IP3-sensitive internal stores in a green monkey kidney cell line. J Physiol 493:371–384
Juul CA, Grubb S, Poulsen KA, Kyed T, Hashem N, Lambert IH, Larsen EH, Hoffmann EK (2014) Anoctamin 6 differs from VRAC and VSOAC but is involved in apoptosis and supports volume regulation in the presence of Ca. Pflugers Arch 466:1899–1910
Klausen TK, Bergdahl A, Hougaard C, Christophersen P, Pedersen SF, Hoffmann EK (2007) Cell cycle-dependent activity of the volume- and Ca2+-activated anion currents in Ehrlich lettre ascites cells. J Cell Physiol 210:831–842
Kmit A, van Kruchten R, Ousingsawat J, Mattheij NJ, Senden-Gijsbers B, Heemskerk JW, Bevers EM, Kunzelmann K (2013) Calcium-activated and apoptotic phospholipid scrambling induced by Ano6 can occur independently of Ano6 ion currents. Cell Death Dis 4:e611
Kunzelmann K, Tian Y, Martins JR, Faria D, Kongsuphol P, Ousingsawat J, Thevenod F, Roussa E, Rock JR, Schreiber R (2011) Anoctamins. Pflugers Arch 462:195–208
Lee MY, Song H, Nakai J, Ohkura M, Kotlikoff MI, Kinsey SP, Golovina VA, Blaustein MP (2006) Local subplasma membrane Ca2+ signals detected by a tethered Ca2+ sensor. Proc Natl Acad Sci U S A 103:13232–13237
Lehtonen JY, Kinnunen PK (1995) Phospholipase A2 as a mechanosensor. Biophys J 68:1888–1894
Lemonnier L, Prevarskaya N, Shuba Y, Vanden Abeele F, Nilius B, Mazurier J, Skryma R (2002) Ca2+ modulation of volume-regulated anion channels: evidence for colocalization with store-operated channels. FASEB J 16:222–224
Malvezzi M, Chalat M, Janjusevic R, Picollo A, Terashima H, Menon AK, Accardi A (2013) Ca(2+)-dependent phospholipid scrambling by a reconstituted TMEM16 ion channel. Nat Commun 4:2367
Martins JR, Faria D, Kongsuphol P, Reisch B, Schreiber R, Kunzelmann K (2011) Anoctamin 6 is an essential component of the outwardly rectifying chloride channel. Proc Natl Acad Sci U S A 108:18168–18172
McCarty NA, O'Neil RG (1992) Calcium signalling in volume regulation. Physiol Rev 72:1037–1061
Mohanty MJ, Ye M, Li X, Rossi NF (2001) Hypotonic swelling-induced Ca(2+) release by an IP(3)-insensitive Ca(2+) store. Am J Physiol Cell Physiol 281:C555–C562
Murakami M, Kudo I (2002) Phospholipase A2. J Biochem 131:285–292
Namkung W, Phuan PW, Verkman AS (2011) TMEM16A inhibitors reveal TMEM16A as a minor component of CaCC conductance in airway and intestinal epithelial cells. J Biol Chem 286:2365–2374
Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G (1997) Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol 68:69–119
Nilius B, Eggermont J, Voets T, Droogmans G (1996) Volume-activated Cl-channels. Gen Pharmacol 27:1131–1140
Nilius B, Oike M, Zahradnik I, Droogmans G (1994) Activation of a Cl-current by hypotonic volume increase in human endothelial cells. J Gen Physiol 103:787–805
Okada Y (2006) Cell volume-sensitive chloride channels: phenotypic properties and molecular identity. Contrib Nephrol 152:9–24
Ousingsawat J, Martins JR, Schreiber R, Rock JR, Harfe BD, Kunzelmann K (2009) Loss of TMEM16A causes a defect in epithelial Ca2+ dependent chloride transport. J Biol Chem 284:28698–28703
Ousingsawat J, Wanitchakool P, Kmit A, Romao AM, Jantarajit W, Schreiber S, Kunzelmann K (2015) Anoctamin 6 mediates effects essential for innate immunity downstream of P2X7-receptors in macrophages. Nat Commun 6:6245
Pedemonte N, Galietta LJ (2014) Structure and function of TMEM16 proteins (anoctamins). Physiol Rev 94:419–459
Pedersen S, Lambert IH, Thoroed SM, Hoffmann EK (2000) Hypotonic cell swelling induces translocation of the alpha isoform of cytosolic phospholipase A2 but not the gamma isoform in Ehrlich ascites tumor cells. Eur J Biochem 267:5531–5539
Pedersen SF, Nilius B (2007) Transient receptor potential channels in mechanosensing and cell volume regulation. Methods Enzymol 428:183–207
Pedersen SF, Poulsen KA, Lambert IH (2006) Roles of phospholipase A2 isoforms in swelling- and melittin-induced arachidonic acid release and taurine efflux in NIH3T3 fibroblasts. Am J Physiol Cell Physiol 291:C1286–C1296
Preston GM, Carroll TP, Guggino WB, Agre P (1992) Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256:385–387
Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A (2014) SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 157:447–458
Sabirov RZ, Prenen J, Tomita T, Droogmans G, Nilius B (2000) Reduction of ionic strength activates single volume-regulated anion channels (VRAC) in endothelial cells. Pflugers Arch 439:315–320
Schreiber R, Faria D, Skryabin BV, Rock JR, Kunzelmann K (2014) Anoctamins support calcium-dependent chloride secretion by facilitating calcium signaling in adult mouse intestine. Pflugers Arch 467:1203–1213
Shimizu T, Iehara T, Sato K, Fujii T, Sakai H, Okada Y (2013) TMEM16F is a component of a Ca2+-activated Cl-channel but not a volume-sensitive outwardly rectifying Cl-channel. Am J Physiol Cell Physiol 304:C748–C759
Strange K, Emma F, Jackson PS (1996) Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol 270:C711–C730
Suzuki J, Umeda M, Sims PJ, Nagata S (2010) Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468:834–838
Szteyn K, Schmid E, Nurbaeva MK, Yang W, Munzer P, Kunzelmann K, Lang F, Shumilina E (2012) Expression and functional significance of the Ca-activated Cl(−) channel ANO6 in dendritic cells. Cell Physiol Biochem 30:1319–1332
Thoroed SM, Lauritzen L, Lambert IH, Hansen HS, Hoffmann EK (1997) Cell swelling activates phospholipase A2 in Ehrlich ascites tumor cells. J Membr Biol 160:47–58
Tian Y, Schreiber R, Kunzelmann K (2012) Anoctamins are a family of Ca2+ activated Cl-channels. J Cell Sci 125:4991–4998
Voets T, Droogmans G, Raskin G, Eggermont J, Nilius B (1999) Reduced intracellular ionic strength as the initial trigger for activation of endothelial volume-regulated anion channels. Proc Natl Acad Sci U S A 96:5298–5303
Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ (2014) Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 344:634–638
Yang H, Kim A, David T, Palmer D, Jin T, Tien J, Huang F, Cheng T, Coughlin SR, Jan YN, Jan LY (2012) TMEM16F forms a Ca(2+)-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell 151:111–122
Yoo J, Cui Q (2009) Curvature generation and pressure profile modulation in membrane by lysolipids: insights from coarse-grained simulations. Biophys J 97:2267–2276
Yu K, Whitlock JM, Lee K, Ortlund EA, Yuan CY, and Hartzell HC (2015) Identification of a lipid scrambling domain in ANO6/TMEM16F. Elife. 4. doi: 10
Zholos A, Beck B, Sydorenko V, Lemonnier L, Bordat P, Prevarskaya N, Skryma R (2005) Ca(2+)- and volume-sensitive chloride currents are differentially regulated by agonists and store-operated Ca2+ entry. J Gen Physiol 125:197–211
Acknowledgments
This study was supported by DFG SFB699-A7/A12, DFG KU756/12-1, Sander-Stiftung 2013.031.1 and Volkswagenstiftung AZ 87 499. We thank Dr. Johan Heemskerk and Dr. Eduard Bevers for supplying the lymphocyte cell lines. The excellent technical assistance by Mss. B. Wild, P. Seeberger, E. Tartler, Inês Cabrita and Mr. Simon Höllerer is gratefully acknowledged.
Author contribution statement
L.S., J.O., P.W. and R.S. performed the experiments. L.S., J.O., P.W., R.S. and K.K. wrote the manuscript and prepared the figures. L.S., J.O., P.W., R.S. and K.K. reviewed the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
All animal experiments were approved by the local ethics commission of the University of Regensburg and were conducted according to the guidelines of the American Physiological Society and the German law for welfare of animals.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Lalida Sirianant, Jiraporn Ousingsawat and Podchanart Wanitchakool shared first authorship.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
(PDF 98 kb)
Supplementary Fig. 2
(PDF 323 kb)
Rights and permissions
About this article
Cite this article
Sirianant, L., Ousingsawat, J., Wanitchakool, P. et al. Cellular volume regulation by anoctamin 6: Ca2+, phospholipase A2 and osmosensing. Pflugers Arch - Eur J Physiol 468, 335–349 (2016). https://doi.org/10.1007/s00424-015-1739-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-015-1739-8