Abstract
The hexanucleotide repeat expansion GGGGCC (G4C2)n in the C9orf72 gene is the most common genetic abnormality associated with amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Recent findings suggest that dysfunction of nuclear-cytoplasmic trafficking could affect the transport of RNA binding proteins in C9orf72 ALS/FTD. Here, we provide evidence that the RNA editing enzyme adenosine deaminase acting on RNA 2 (ADAR2) is mislocalized in C9orf72 repeat expansion mediated ALS/FTD. ADAR2 is responsible for adenosine (A) to inosine (I) editing of double-stranded RNA, and its function has been shown to be essential for survival. Here we show the mislocalization of ADAR2 in human induced pluripotent stem cell-derived motor neurons (hiPSC-MNs) from C9orf72 patients, in mice expressing (G4C2)149, and in C9orf72 ALS/FTD patient postmortem tissue. As a consequence of this mislocalization we observe alterations in RNA editing in our model systems and across multiple brain regions. Analysis of editing at 408,580 known RNA editing sites indicates that there are vast RNA A to I editing aberrations in C9orf72-mediated ALS/FTD. These RNA editing aberrations are found in many cellular pathways, such as the ALS pathway and the crucial EIF2 signaling pathway. Our findings suggest that the mislocalization of ADAR2 in C9orf72 mediated ALS/FTD is responsible for the alteration of RNA processing events that may impact vast cellular functions, including the integrated stress response (ISR) and protein translation.







Similar content being viewed by others
Change history
26 September 2019
The original article was published erroneously without mentioning the support of the U.S.
References
Akamatsu M, Yamashita T, Hirose N, Teramoto S, Kwak S (2016) The AMPA receptor antagonist perampanel robustly rescues amyotrophic lateral sclerosis (ALS) pathology in sporadic ALS model mice. Sci Rep 6:28649. https://doi.org/10.1038/srep28649
Almeida S, Gascon E, Tran H, Chou HJ, Gendron TF, Degroot S et al (2013) Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC-derived human neurons. Acta Neuropathol 126:385–399. https://doi.org/10.1007/s00401-013-1149-y
Ash Peter EA, Bieniek Kevin F, Gendron Tania F, Caulfield T, Lin W-L, DeJesus-Hernandez M et al (2013) Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 77:639–646. https://doi.org/10.1016/j.neuron.2013.02.004
Atanasio A, Decman V, White D, Ramos M, Ikiz B, Lee HC et al (2016) C9orf72 ablation causes immune dysregulation characterized by leukocyte expansion, autoantibody production, and glomerulonephropathy in mice. Sci Rep 6:23204. https://doi.org/10.1038/srep23204
Bass BL, Weintraub H (1987) A developmentally regulated activity that unwinds RNA duplexes. Cell 48:607–613. https://doi.org/10.1016/0092-8674(87)90239-X
Behm M, Wahlstedt H, Widmark A, Eriksson M, Ohman M (2017) Accumulation of nuclear ADAR2 regulates adenosine-to-inosine RNA editing during neuronal development. J Cell Sci 130:745–753. https://doi.org/10.1242/jcs.200055
Bell MC, Meier SE, Ingram AL, Abisambra JF (2016) PERK-opathies: an endoplasmic reticulum stress mechanism underlying neurodegeneration. Curr Alzheimer Res 13:150–163
Belzil VV, Bauer PO, Prudencio M, Gendron TF, Stetler CT, Yan IK et al (2013) Reduced C9orf72 gene expression in c9FTD/ALS is caused by histone trimethylation, an epigenetic event detectable in blood. Acta Neuropathol 126:895–905. https://doi.org/10.1007/s00401-013-1199-1
Boeynaems S, Bogaert E, Michiels E, Gijselinck I, Sieben A, Jovičić A et al (2016) Drosophila screen connects nuclear transport genes to DPR pathology in c9ALS/FTD. Sci Rep 6: 20877. https://doi.org/10.1038/srep20877. http://www.nature.com/articles/srep20877—supplementary-information
Boeynaems S, Bogaert E, Van Damme P, Van Den Bosch L (2016) Inside out: the role of nucleocytoplasmic transport in ALS and FTLD. Acta Neuropathol 132:159–173. https://doi.org/10.1007/s00401-016-1586-5
Burberry A, Suzuki N, Wang J-Y, Moccia R, Mordes DA, Stewart MH et al (2016) Loss-of-function mutations in the C9ORF72 mouse ortholog cause fatal autoimmune disease. Sci Transl Med 8:347ra393
Cesarini V, Silvestris DA, Tassinari V, Tomaselli S, Alon S, Eisenberg E et al (2017) ADAR2/miR-589-3p axis controls glioblastoma cell migration/invasion. Nucleic Acids Res. https://doi.org/10.1093/nar/gkx1257
Chen CX, Cho DS, Wang Q, Lai F, Carter KC, Nishikura K (2000) A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA 6:755–767
Cheng W, Wang S, Mestre AA, Fu C, Makarem A, Xian F et al (2018) C9ORF72 GGGGCC repeat-associated non-AUG translation is upregulated by stress through eIF2α phosphorylation. Nat Commun 9:51. https://doi.org/10.1038/s41467-017-02495-z
Chesnokova E, Bal N, Kolosov P (2017) Kinases of eIF2a switch translation of mRNA subset during neuronal plasticity. Int J Mol Sci. https://doi.org/10.3390/ijms18102213
Chew J, Cook C, Gendron TF, Jansen-West K, Del Rosso G, Daughrity LM et al (2019) Aberrant deposition of stress granule-resident proteins linked to C9orf72-associated TDP-43 proteinopathy. Mol Neurodegener 14:9. https://doi.org/10.1186/s13024-019-0310-z
Ciura S, Lattante S, Le Ber I, Latouche M, Tostivint H, Brice A et al (2013) Loss of function of C9orf72 causes motor deficits in a zebrafish model of amyotrophic lateral sclerosis. Ann Neurol 74:180–187. https://doi.org/10.1002/ana.23946
Connell MA, Krause S, Higuchi M, Hsuan JJ, Totty NF, Jenny A et al (1995) Cloning of cDNAs encoding mammalian double-stranded RNA-specific adenosine deaminase. Mol Cell Biol 15:1389
DeJesus-Hernandez M, Mackenzie Ian R, Boeve Bradley F, Boxer Adam L, Baker M, Rutherford Nicola J et al (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256. https://doi.org/10.1016/j.neuron.2011.09.011
Devi L, Ohno M (2014) PERK mediates eIF2α phosphorylation responsible for BACE1 elevation, CREB dysfunction and neurodegeneration in a mouse model of Alzheimer’s disease. Neurobiol Aging 35:2272–2281. https://doi.org/10.1016/j.neurobiolaging.2014.04.031
Donnelly CJ, Zhang PW, Pham JT, Heusler AR, Mistry NA, Vidensky S et al (2013) RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron 80:415–428. https://doi.org/10.1016/j.neuron.2013.10.015
Eftekharzadeh B, Daigle JG, Kapinos LE, Coyne A, Schiantarelli J, Carlomagno Y et al (2018) Tau protein disrupts nucleocytoplasmic transport in Alzheimer’s disease. Neuron 99(925–940):e927. https://doi.org/10.1016/j.neuron.2018.07.039
Franzén O, Ermel R, Sukhavasi K, Jain R, Jain A, Betsholtz C et al (2018) Global analysis of A-to-I RNA editing reveals association with common disease variants. PeerJ 6:e4466. https://doi.org/10.7717/peerj.4466
Freibaum BD, Lu Y, Lopez-Gonzalez R, Kim NC, Almeida S, Lee K-H et al (2015) GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature 525:129. https://doi.org/10.1038/nature14974. http://www.nature.com/articles/nature14974—supplementary-information
Gasset-Rosa F, Chillon-Marinas C, Goginashvili A, Atwal RS, Artates JW, Tabet R et al (2017) Polyglutamine-expanded huntingtin exacerbates age-related disruption of nuclear integrity and nucleocytoplasmic transport. Neuron 94(48–57):e44. https://doi.org/10.1016/j.neuron.2017.03.027
Gendron TF, van Blitterswijk M, Bieniek KF, Daughrity LM, Jiang J, Rush BK et al (2015) Cerebellar c9RAN proteins associate with clinical and neuropathological characteristics of C9ORF72 repeat expansion carriers. Acta Neuropathol 130:559–573. https://doi.org/10.1007/s00401-015-1474-4
Gijselinck I, Van Langenhove T, van der Zee J, Sleegers K, Philtjens S, Kleinberger G et al (2012) A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum: a gene identification study. Lancet Neurol 11:54–65. https://doi.org/10.1016/S1474-4422(11)70261-7
Grima JC, Daigle JG, Arbez N, Cunningham KC, Zhang K, Ochaba J et al (2017) Mutant huntingtin disrupts the nuclear pore complex. Neuron 94(93–107):e106. https://doi.org/10.1016/j.neuron.2017.03.023
Haeusler AR, Donnelly CJ, Rothstein JD (2016) The expanding biology of the C9orf72 nucleotide repeat expansion in neurodegenerative disease. Nat Rev Neurosci 17:383. https://doi.org/10.1038/nrn.2016.38. http://www.nature.com/articles/nrn.2016.38—supplementary-information
Hartner JC, Schmittwolf C, Kispert A, Muller AM, Higuchi M, Seeburg PH (2004) Liver disintegration in the mouse embryo caused by deficiency in the RNA-editing enzyme ADAR1. J Biol Chem 279:4894–4902. https://doi.org/10.1074/jbc.M311347200
Hetz C, Mollereau B (2014) Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci 15:233. https://doi.org/10.1038/nrn3689
Hideyama T, Yamashita T, Aizawa H, Tsuji S, Kakita A, Takahashi H et al (2012) Profound downregulation of the RNA editing enzyme ADAR2 in ALS spinal motor neurons. Neurobiol Dis 45:1121–1128. https://doi.org/10.1016/j.nbd.2011.12.033
Hideyama T, Yamashita T, Suzuki T, Tsuji S, Higuchi M, Seeburg PH et al (2010) Induced loss of ADAR2 engenders slow death of motor neurons from Q/R site-unedited GluR2. J Neurosci 30:11917–11925. https://doi.org/10.1523/JNEUROSCI.2021-10.2010
Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N et al (2000) Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature 406:78–81. https://doi.org/10.1038/35017558
Hwang T, Park CK, Leung AK, Gao Y, Hyde TM, Kleinman JE et al (2016) Dynamic regulation of RNA editing in human brain development and disease. Nat Neurosci 19:1093–1099. https://doi.org/10.1038/nn.4337
Jackson RJ, Hellen CUT, Pestova TV (2010) The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol 11:113. https://doi.org/10.1038/nrm2838. http://www.nature.com/articles/nrm2838—supplementary-information
Jovičić A, Mertens J, Boeynaems S, Bogaert E, Chai N, Yamada SB et al (2015) Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS. Nat Neurosci 18:1226. https://doi.org/10.1038/nn.4085. http://www.nature.com/articles/nn.4085—supplementary-information
Kim U, Wang Y, Sanford T, Zeng Y, Nishikura K (1994) Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc Natl Acad Sci 91:11457
Kwak S, Kawahara Y (2005) Deficient RNA editing of GluR2 and neuronal death in amyotropic lateral sclerosis. J Mol Med 83:110–120. https://doi.org/10.1007/s00109-004-0599-z
Lagier-Tourenne C, Baughn M, Rigo F, Sun S, Liu P, Li H-R et al (2013) Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc Natl Acad Sci 110:E4530
Lee Y-B, Chen H-J, Peres João N, Gomez-Deza J, Attig J, Štalekar M et al (2013) Hexanucleotide repeats in ALS/FTD form length-dependent RNA foci, sequester RNA binding proteins, and are neurotoxic. Cell Rep 5:1178–1186. https://doi.org/10.1016/j.celrep.2013.10.049
Lorenzini I, Moore S, Sattler R (2018) RNA editing deficiency in neurodegeneration. Adv Neurobiol 20:63–83. https://doi.org/10.1007/978-3-319-89689-2_3
Maemura K, Watanabe K, Ando T, Hiyama N, Sakatani T, Amano Y et al (2018) Altered editing level of microRNAs is a potential biomarker in lung adenocarcinoma. Cancer Sci. https://doi.org/10.1111/cas.13742
May S, Hornburg D, Schludi MH, Arzberger T, Rentzsch K, Schwenk BM et al (2014) C9orf72 FTLD/ALS-associated Gly-Ala dipeptide repeat proteins cause neuronal toxicity and Unc119 sequestration. Acta Neuropathol 128:485–503. https://doi.org/10.1007/s00401-014-1329-4
Melcher T, Maas S, Higuchi M, Keller W, Seeburg PH (1995) Editing of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR-B pre-mRNA in vitro reveals site-selective adenosine to inosine conversion. J Biol Chem 270:8566–8570
Mizielinska S, Grönke S, Niccoli T, Ridler CE, Clayton EL, Devoy A et al (2014) C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science 345:1192
Mohan A, Goodwin M, Swanson MS (2014) RNA–protein interactions in unstable microsatellite diseases. Brain Res 1584:3–14. https://doi.org/10.1016/j.brainres.2014.03.039
Mori K, Weng S-M, Arzberger T, May S, Rentzsch K, Kremmer E et al (2013) The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science 339:1335
Orecchini E, Frassinelli L, Galardi S, Ciafre SA, Michienzi A (2018) Post-transcriptional regulation of LINE-1 retrotransposition by AID/APOBEC and ADAR deaminases. Chromosome Res Int J Mol Supramol Evol Asp Chromosome Biol 26:45–59. https://doi.org/10.1007/s10577-018-9572-5
Paonessa F, Evans LD, Solanki R, Larrieu D, Wray S, Hardy J et al (2019) Microtubules deform the nuclear membrane and disrupt nucleocytoplasmic transport in tau-mediated frontotemporal dementia. Cell Rep 26(582–593):e585. https://doi.org/10.1016/j.celrep.2018.12.085
Pfaller CK, Li Z, George CX, Samuel CE (2011) Protein kinase PKR and RNA adenosine deaminase ADAR1: new roles for old players as modulators of the interferon response. Curr Opin Immunol 23:573–582. https://doi.org/10.1016/j.coi.2011.08.009
Reed SE, Staley EM, Mayginnes JP, Pintel DJ, Tullis GE (2006) Transfection of mammalian cells using linear polyethylenimine is a simple and effective means of producing recombinant adeno-associated virus vectors. J Virol Methods 138:85–98. https://doi.org/10.1016/j.jviromet.2006.07.024
Renton Alan E, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR et al (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72:257–268. https://doi.org/10.1016/j.neuron.2011.09.010
Sareen D, O’Rourke JG, Meera P, Muhammad AKMG, Grant S, Simpkinson M et al (2013) Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci Transl Med 5:208ra149
Selvaraj BT, Livesey MR, Zhao C, Gregory JM, James OT, Cleary EM et al (2018) C9ORF72 repeat expansion causes vulnerability of motor neurons to Ca2+-permeable AMPA receptor-mediated excitotoxicity. Nat Commun 9:347. https://doi.org/10.1038/s41467-017-02729-0
Sharpnack MF, Chen B, Aran D, Kosti I, Sharpnack DD, Carbone DP et al (2018) Global transcriptome analysis of RNA abundance regulation by ADAR in lung adenocarcinoma. EBioMedicine 27:167–175. https://doi.org/10.1016/j.ebiom.2017.12.005
Shelton PM, Duran A, Nakanishi Y, Reina-Campos M, Kasashima H, Llado V et al (2018) The secretion of miR-200s by a PKCζ/ADAR2 signaling axis promotes liver metastasis in colorectal cancer. Cell Rep 23:1178–1191. https://doi.org/10.1016/j.celrep.2018.03.118
Shi Y, Lin S, Staats KA, Li Y, Chang W-H, Hung S-T et al (2018) Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat Med 24:313. https://doi.org/10.1038/nm.4490. http://www.nature.com/articles/nm.4490—supplementary-information
Sun W, Samimi H, Gamez M, Zare H, Frost B (2018) Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies. Nat Neurosci 21:1038–1048. https://doi.org/10.1038/s41593-018-0194-1
Takuma H, Kwak S, Yoshizawa T, Kanazawa I (1999) Reduction of GluR2 RNA editing, a molecular change that increases calcium influx through AMPA receptors, selective in the spinal ventral gray of patients with amyotrophic lateral sclerosis. Ann Neurol 46:806–815
Tan MH, Li Q, Shanmugam R, Piskol R, Kohler J, Young AN et al (2017) Dynamic landscape and regulation of RNA editing in mammals. Nature 550:249–254. https://doi.org/10.1038/nature24041
Therrien M, Rouleau GA, Dion PA, Parker JA (2013) Deletion of C9ORF72 results in motor neuron degeneration and stress sensitivity in C. elegans. PLOS ONE 8:e83450. https://doi.org/10.1371/journal.pone.0083450
Tran H, Almeida S, Moore J, Gendron Tania F, Chalasani U, Lu Y et al (2015) Differential toxicity of nuclear RNA foci versus dipeptide repeat proteins in a Drosophila model of C9ORF72 FTD/ALS. Neuron 87:1207–1214. https://doi.org/10.1016/j.neuron.2015.09.015
Tran SS, Jun HI, Bahn JH, Azghadi A, Ramaswami G, Van Nostrand EL et al (2019) Widespread RNA editing dysregulation in brains from autistic individuals. Nat Neurosci 22:25–36. https://doi.org/10.1038/s41593-018-0287-x
Vucic S, Rothstein JD, Kiernan MC (2014) Advances in treating amyotrophic lateral sclerosis: insights from pathophysiological studies. Trends Neurosci 37:433–442. https://doi.org/10.1016/j.tins.2014.05.006
Waite AJ, Bäumer D, East S, Neal J, Morris HR, Ansorge O et al (2014) Reduced C9orf72 protein levels in frontal cortex of amyotrophic lateral sclerosis and frontotemporal degeneration brain with the C9ORF72 hexanucleotide repeat expansion. Neurobiol Aging 35:1779.e5–1779.e13. https://doi.org/10.1016/j.neurobiolaging.2014.01.016
Wang Q, Khillan J, Gadue P, Nishikura K (2000) Requirement of the RNA editing deaminase ADAR1 gene for embryonic erythropoiesis. Science 290:1765
Wang Q, Miyakoda M, Yang W, Khillan J, Stachura DL, Weiss MJ et al (2004) Stress-induced apoptosis associated with null mutation of ADAR1 RNA editing deaminase gene. J Biol Chem 279:4952–4961. https://doi.org/10.1074/jbc.M310162200
Wen X, Tan W, Westergard T, Krishnamurthy K, Markandaiah SS, Shi Y et al (2014) Antisense proline-arginine RAN dipeptides linked to C9ORF72-ALS/FTD form toxic nuclear aggregates that initiate in vitro and in vivo neuronal death. Neuron 84:1213–1225. https://doi.org/10.1016/j.neuron.2014.12.010
Xiao S, MacNair L, McGoldrick P, McKeever PM, McLean JR, Zhang M et al (2015) Isoform-specific antibodies reveal distinct subcellular localizations of C9orf72 in amyotrophic lateral sclerosis. Ann Neurol 78:568–583. https://doi.org/10.1002/ana.24469
Yang D, Abdallah A, Li Z, Lu Y, Almeida S, Gao F-B (2015) FTD/ALS-associated poly(GR) protein impairs the Notch pathway and is recruited by poly(GA) into cytoplasmic inclusions. Acta Neuropathol 130:525–535. https://doi.org/10.1007/s00401-015-1448-6
Yu W, Xu H, Xue Y, An D, Li H, Chen W et al (2018) 5-HT2CR antagonist/5-HT2CR inverse agonist recovered the increased isolation-induced aggressive behavior of BALB/c mice mediated by ADAR1 (p110) expression and Htr2c RNA editing. Brain Behav 8:e00929. https://doi.org/10.1002/brb3.929
Zhang K, Donnelly CJ, Haeusler AR, Grima JC, Machamer JB, Steinwald P et al (2015) The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature 525:56. https://doi.org/10.1038/nature14973. http://www.nature.com/articles/nature14973—supplementary-information
Zhang Y-J, Gendron TF, Ebbert MTW, O’Raw AD, Yue M, Jansen-West K et al (2018) Poly(GR) impairs protein translation and stress granule dynamics in C9orf72-associated frontotemporal dementia and amyotrophic lateral sclerosis. Nat Med 24:1136–1142. https://doi.org/10.1038/s41591-018-0071-1
Zhang Y-J, Jansen-West K, Xu Y-F, Gendron TF, Bieniek KF, Lin W-L et al (2014) Aggregation-prone c9FTD/ALS poly(GA) RAN-translated proteins cause neurotoxicity by inducing ER stress. Acta Neuropathol 128:505–524. https://doi.org/10.1007/s00401-014-1336-5
Zu T, Liu Y, Bañez-Coronel M, Reid T, Pletnikova O, Lewis J et al (2013) RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Natl Acad Sci 110:E4968
Acknowledgements
We would like to thank the Sattler Laboratory for suggestions and comments towards the manuscript. We would also like to thank all ALS patients and families that have contributed to this research via postmortem brain tissue donations. Specifically, we would like to thank Doug Clough for assistance with data analysis and insightful discussions. We further thank the Target ALS Human Postmortem Tissue Core, New York Genome Center for Genomics of Neurodegenerative Disease, Amyotrophic Lateral Sclerosis Association and TOW Foundation for providing access to their postmortem patient tissue samples collection. We thank both the Target ALS Consortium and the New York Genome Center for access to their RNA sequencing database. In particular, we would like to thank Drs. Lyle Ostrow, Hemali Phatnani and Robert Bowser. We would also like to thank Drs. Sylvia Perez and Elliott Mufson for generously providing us with AD patient postmortem tissue samples. Further thanks go to Dr. Stella Dracheva for helpful discussions throughout this project. This work was support by the National Institute of Neurological Disorders and Stroke, NIH RO1NS085207 (RS); the Muscular Dystrophy Association (RS); the ALS Association (RS); the Robert Packard Center for ALS Research (RS); and the Barrow Neurological Foundation (RS). Part of this work was also made possible by NIH Grant R01NS097850 (JKI), US Department of Defense Grant W81XWH-15-1-0187 (JI), and grants from the Donald E. and Delia B. Baxter Foundation (JKI), the Alzheimer’s Drug Discovery Foundation (JKI) and the Association for Frontotemporal Degeneration (JKI), the Harrington Discovery Institute (JKI), the Tau Consortium (JKI), the Pape Adams Foundation (JKI), the Frick Foundation for ALS Research (JKI), the Muscular Dystrophy Association (JKI), the New York Stem Cell Foundation (JKI), the USC Keck School of Medicine Regenerative Medicine Initiative (JKI), the USC Broad Innovation Award (JKI), and the Southern California Clinical and Translational Science Institute to JKI. JKI is a New York Stem Cell Foundation-Robertson Investigator and a Richard N. Merkin Scholar. We would additionally like to thank the National Institutes of Health/National Institute of Neurological Disorders and Stroke [R35NS097273 (L.P.); P01NS084974 (L.P.); P01NS099114 (L.P.); R01NS088689 (L.P.)]; the Mayo Clinic Foundation (L.P.); the Amyotrophic Lateral Sclerosis Association (L.P.), the Robert Packard Center for ALS Research at Johns Hopkins (L.P.), the Target ALS Foundation (L.P.), and the James Hunter Family ALS Initiative (JR).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1. Structure and Domains of ADAR1, ADAR2, and ADAR3.
(a) ADAR1 can commonly be referred to ADAR, contains a nuclear export sequence, 2 Z-DNA binding domains, 3 double-stranded RNA binding domains, a nuclear localization sequence and a catalytically active deaminase domain. (b) ADAR2 sometimes referred to as ADARb1 only contains a nuclear localization signal and is confined to the nucleus, it has two double-stranded RNA binding domains and a catalytically active deaminase domain. (c) ADAR3 sometimes referred to ADARb2 is the only member of the ADAR family thought to be catalytically inactive. (TIFF 3488 kb)
Online Resource 2. Demographics of Patient Postmortem Tissue, hiPSC Lines, and RNA Samples from the NYGC.
We received C9orf72 ALS/FTD and non-ALS postmortem spinal cord, motor cortex, and cerebellum for immunohistochemistry from both Dr. Janice Robertson as well as Target ALS. hiPSC motor neurons were differentiated in Dr. Rita Sattler’s lab and Dr. Justin Ichida’s lab, these neurons were used for immunocytochemistry, RNA sequencing, and RNA editing analysis. We utilized RNA sequencing of C9orf72 ALS/FTD spinal cord, motor cortex, frontal cortex, and cerebellum performed by the NYGC to analyze and characterize RNA editing in postmortem C9orf72 ALS/FTD patient tissue. (XLSX 19 kb)
Online Resource 3. RNA A to I Editing Sites Analyzed for Editing Aberration.
Complete list of sites determined to display A to I RNA editing that satisfied our requirements of a coverage of 20x across half the non-ALS controls and half the C9orf72 ALS/FTD analyzed. Also included are all differentially edited A to I editing sites and genes in C9orf72 ALS/FTD tissue, hiPSC motor neurons, siRNA treated hiPSC motor neurons and HEK293 cells expressing nuclear or cytoplasmic ADAR2. (XLSX 8938 kb)
Online Resource 4. ADAR2 Mislocalization in Patient Postmortem Spinal Cord Tissue.
(a) Four additional MAP2 positive neurons as examples of normal ADAR2 mislocalization in non-ALS patient postmortem spinal cord. (b) Four additional MAP2 positive neurons as examples of cytoplasmic accumulations of ADAR2, these images display different types of cytoplasmic accumulations that have been observed in patient postmortem tissue. (c-e) ADAR2 accumulations in the dendrite of a spinal motor neuron of a C9orf72 ALS/FTD patient. (f-j) No primary antibody negative control. (TIFF 37158 kb)
Online Resource 5. Co-pathology of ADAR2 and TDP43.
(a-h) two examples of spinal motor neurons that have dual TDP-43 pathology and cytoplasmic ADAR2 accumulation. Quantification is provided as the percentage of neurons that display both ADAR2 and TDP-43 cytoplasmic accumulation (* p < 0.05). (TIFF 9510 kb)
Online Resource 6. ADAR2 Mislocalization in Patient Postmortem Motor Cortex Tissue.
(a) four additional examples of ADAR2 localization in non-ALS control motor cortex. (b) four additional examples of MAP2 positive neurons in C9orf72 ALS/FTD motor cortex, four neurons displaying ADAR2 cytoplasmic accumulations and one neuron displaying typical nuclear ADAR2 distribution. (Bottom) No primary antibody negative control. (TIFF 19370 kb)
Online Resource 7. ADAR2 is Not Significantly Mislocalized in C9orf72 Frontal Cortex.
(a) 3 neurons from non-ALS control patient postmortem tissue displaying typical nuclear ADAR2 staining. (b) 3 neurons from C9orf72 ALS/FTD patient postmortem tissue displaying cytoplasmic accumulations of ADAR2. (c) No significant difference in the percentage of neurons that display cytoplasmic accumulation of ADAR2. (p < 0.10, t-test). (d – h) No Primary Antibody negative control. (TIFF 18119 kb)
Online Resource 8. ADAR2 Mislocalization in hiPSC Derived Motor Neurons.
(a) 10 motor neurons stained for ADAR2 in healthy control hiPSC-MNs displaying typical nuclear localization. (b) 10 C9orf72 ALS/FTD hiPSC differentiated motor neurons displaying cytoplasmic accumulation of ADAR2. (TIFF 35055 kb)
Online Resource 9. ADAR2 Mislocalization in Severe AD Frontal Cortex.
(a-d) cytoplasmic accumulation of ADAR2 in a patient with mild cognitive impairment. (e–h) patients with mild AD present with limited ADAR2 mislocalization. (i-l) Cytoplasmic accumulation of ADAR2 in a patient with severe AD. (m) Quantification of MAP2 positive neurons in the frontal cortex of MCI, mild AD, and severe AD reveal 20.3% (p = 0.49) of neurons in patients with mild cognitive impairment, 21.5% (p = 0.43) of neurons in patients with mild AD and 39.66%(p = 0.04, ANOVA) of neurons in tissue from severe AD patients. (TIFF 8341 kb)
Online Resource 10. Gene Expression of AMPA Receptor Subunits (
GluA1-4) andADAR 1-3in C9orf72 ALS/FTD Human Postmortem Tissue and hiPSC Motor Neurons. Gene expression from RNA sequencing performed by the NYGC on C9orf72 ALS/FTD human postmortem tissue was compared to control human postmortem tissue and gene expression of C9orf72 hiPSC motor neurons was compared to control hiPSC neurons. Fold change gene expression of the AMPA receptor and ADAR family collected from RNA sequencing of C9orf72 ALS/FTD tissues and hiPSC derived motor neurons compared to controls. (**, p = 0.01, t-test) (TIFF 4797 kb)
Online Resource 11. Volcano Plots of RNA Editing Aberrations in Other Human Tissues.
(a-c) Volcano plots showing the spread of all RNA editing aberrations in (a) motor cortex, (b) frontal cortex, and (c) cerebellum. (TIFF 14962 kb)
Online Resource 12. RNA Editing Aberrations in C9orf72 ALS/FTD derived hiPSC Motor Neurons.
RNA sequencing was performed on C9orf72 ALS/FTD hiPSC derived motor neurons by Kim Staats at USC (a) Volcano plot depicting the spread of all RNA editing aberrations in C9orf72 ALS/FTD hiPSC derived neurons. (b) Top hits of all RNA editing aberrations detected in C9orf72 ALS/FTD hiPSC motor neurons. (TIFF 10366 kb)
Online Resource 13. Classification of all Detected RNA Editing Aberrations.
Genomic alignment of RNA A to I editing sites detected in our analysis reveals that the majority of sites are contained in introns, 3′ UTR, non-coding transcripts, relatively few sites are in coding regions that lead to missense variations (1%). (TIFF 5977 kb)
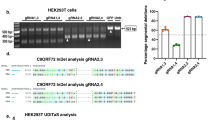
Online Resource 14. siRNA Knockdown of ADAR 1 and 2 Disrupts RNA Editing.
(a) hiPSC motor neurons show successful knockdown after treatment with ADAR1 siRNA in isolation and multiplexed with ADAR2 siRNA. (b) hiPSC motor neurons show successful knockdown after treatment with ADAR2 siRNA in isolation and multiplexed with ADAR1 siRNA. (c) Knockdown of ADAR1 leads to primarily hypo-editing. (d) Knockdown of ADAR2 leads to hyper- and hypo-editing. (e) Knockdown of both ADAR1 and ADAR2 leads to hypo-editing. (TIFF 24682 kb)
Online Resource 15. Tissue Specific and Overlapping RNA Editing Aberrations in C9orf72 ALS/FTD.
Each tab displays a list of genes that are differentially edited in C9orf72 ALS/FTD in each specific tissue and overlapping areas, corresponding to the Venn diagram in Fig. 7a. (XLSX 130 kb)
Online Resource 16. The GluA2 Q/R Site is Mis-Edited in C9orf72 ALS/FTD Postmortem Spinal Cord Tissue.
RNA sequencing analysis of the GluA2 Q/R site reveals modest, yet significant A to I editing differences in C9orf72 ALS/FTD spinal cord and motor cortex, but no differences in frontal cortex and cerebellum. (TIFF 4392 kb)
Online Resource 17. Direct Comparison of Genes with RNA editing Aberrations in Different Models of RNA Editing Dysregulation.
(a) Venn Diagram displaying the common and unique genes which exhibit RNA editing alterations in HEK293T cells overexpressing ΔNLS-ADAR2, C9orf72 ALS/FTD, C9orf72 ALS/FTD hiPSC MNs, and hiPSC MNs treated with ADAR2 siRNA. (b) Genes of interest that are misedited in all different models of abnormal RNA editing. (TIFF 16873 kb)
Online Resource 18. Tissue and Cell Model Specific and Overlapping RNA Editing Aberrations.
Each tab displays a list of genes that are differentially edited in each specific tissue and cell model as well as overlapping areas, corresponding to the Venn diagram in Online Resource 17. (XLSX 203 kb)
Rights and permissions
About this article
Cite this article
Moore, S., Alsop, E., Lorenzini, I. et al. ADAR2 mislocalization and widespread RNA editing aberrations in C9orf72-mediated ALS/FTD. Acta Neuropathol 138, 49–65 (2019). https://doi.org/10.1007/s00401-019-01999-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-019-01999-w