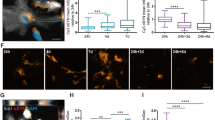
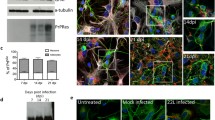
Abstract
Recent evidence suggests that disease progression in Parkinson’s disease (PD) could occur by the spreading of α-synuclein (α-syn) aggregates between neurons. Here we studied the role of astrocytes in the intercellular transfer and fate of α-syn fibrils, using in vitro and ex vivo models. α-Syn fibrils can be transferred to neighboring cells; however, the transfer efficiency changes depending on the cell types. We found that α-syn is efficiently transferred from astrocytes to astrocytes and from neurons to astrocytes, but less efficiently from astrocytes to neurons. Interestingly, α-syn puncta are mainly found inside the lysosomal compartments of the recipient cells. However, differently from neurons, astrocytes are able to efficiently degrade fibrillar α-syn, suggesting an active role for these cells in clearing α-syn deposits. Astrocytes co-cultured with organotypic brain slices are able to take up α-syn fibrils from the slices. Altogether our data support a role for astrocytes in trapping and clearing α-syn pathological deposits in PD.









Similar content being viewed by others
References
Abounit S, Bousset L, Loria F et al (2016) Tunneling nanotubes spread fibrillar alpha-synuclein by intercellular trafficking of lysosomes. EMBO J 35:2120–2138. doi:10.15252/embj.201593411
Abounit S, Zurzolo C (2012) Wiring through tunneling nanotubes–from electrical signals to organelle transfer. J Cell Sci 125:1089–1098. doi:10.1242/jcs.083279
Alvarez-Erviti L, Couch Y, Richardson J, Cooper JM, Wood MJ (2011) Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci Res 69:337–342. doi:10.1016/j.neures.2010.12.020
Anderson MA, Burda JE, Ren Y et al (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195–200. doi:10.1038/nature17623
Apetri MM, Harkes R, Subramaniam V, Canters GW, Schmidt T, Aartsma TJ (2016) Direct observation of alpha-synuclein amyloid aggregates in endocytic vesicles of neuroblastoma cells. PLoS One 11:e0153020. doi:10.1371/journal.pone.0153020
Ayers JI, Brooks MM, Rutherford NJ et al (2017) Robust central nervous system pathology in transgenic mice following peripheral injection of alpha-synuclein fibrils. J Virol. doi:10.1128/JVI.02095-16
Bae EJ, Yang NY, Song M et al (2014) Glucocerebrosidase depletion enhances cell-to-cell transmission of alpha-synuclein. Nat Commun 5:4755. doi:10.1038/ncomms5755
Ball G, Demmerle J, Kaufmann R, Davis I, Dobbie IM, Schermelleh L (2015) SIMcheck: a toolbox for successful super-resolution structured illumination microscopy. Sci Rep 5:15915. doi:10.1038/srep15915
Belanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14:724–738. doi:10.1016/j.cmet.2011.08.016
Bousset L, Pieri L, Ruiz-Arlandis G et al (2013) Structural and functional characterization of two alpha-synuclein strains. Nat Commun 4:2575. doi:10.1038/ncomms3575
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Sastre M, Del Tredici K (2007) Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol 114:231–241. doi:10.1007/s00401-007-0244-3
Brahic M, Bousset L, Bieri G, Melki R, Gitler AD (2016) Axonal transport and secretion of fibrillar forms of alpha-synuclein, Abeta42 peptide and HTTExon 1. Acta Neuropathol 131:539–548. doi:10.1007/s00401-016-1538-0
Braidy N, Gai WP, Xu YH et al (2013) Uptake and mitochondrial dysfunction of alpha-synuclein in human astrocytes, cortical neurons and fibroblasts. Transl Neurodegener 2:20. doi:10.1186/2047-9158-2-20
Bruck D, Wenning GK, Stefanova N, Fellner L (2016) Glia and alpha-synuclein in neurodegeneration: a complex interaction. Neurobiol Dis 85:262–274. doi:10.1016/j.nbd.2015.03.003
Brundin P, Melki R, Kopito R (2010) Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol 11:301–307. doi:10.1038/nrm2873
Cavaliere F, Cerf L, Dehay B et al (2017) In vitro alpha-synuclein neurotoxicity and spreading among neurons and astrocytes using Lewy body extracts from Parkinson disease brains. Neurobiol Dis 103:101–112. doi:10.1016/j.nbd.2017.04.011
Costanzo M, Zurzolo C (2013) The cell biology of prion-like spread of protein aggregates: mechanisms and implication in neurodegeneration. Biochem J 452:1–17. doi:10.1042/BJ20121898
Desplats P, Lee HJ, Bae EJ et al (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA 106:13010–13015. doi:10.1073/pnas.0903691106
Di Malta C, Fryer JD, Settembre C, Ballabio A (2012) Astrocyte dysfunction triggers neurodegeneration in a lysosomal storage disorder. Proc Natl Acad Sci USA 109:E2334–E2342. doi:10.1073/pnas.1209577109
Domert J, Sackmann C, Severinsson E et al (2016) Aggregated alpha-synuclein transfer efficiently between cultured human neuron-like cells and localize to lysosomes. PLoS One 11:e0168700. doi:10.1371/journal.pone.0168700
Freeman D, Cedillos R, Choyke S et al (2013) Alpha-synuclein induces lysosomal rupture and cathepsin dependent reactive oxygen species following endocytosis. PLoS One 8:e62143. doi:10.1371/journal.pone.0062143
Freundt EC, Maynard N, Clancy EK et al (2012) Neuron-to-neuron transmission of alpha-synuclein fibrils through axonal transport. Ann Neurol 72:517–524. doi:10.1002/ana.23747
Ghee M, Melki R, Michot N, Mallet J (2005) PA700, the regulatory complex of the 26S proteasome, interferes with alpha-synuclein assembly. FEBS J 272:4023–4033. doi:10.1111/j.1742-4658.2005.04776.x
Han X, Chen M, Wang F et al (2013) Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12:342–353. doi:10.1016/j.stem.2012.12.015
Hansen C, Angot E, Bergstrom AL et al (2011) alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Investig 121:715–725. doi:10.1172/JCI43366
Ji K, Akgul G, Wollmuth LP, Tsirka SE (2013) Microglia actively regulate the number of functional synapses. PLoS One 8:e56293. doi:10.1371/journal.pone.0056293
Kasai T, Tokuda T, Yamaguchi N et al (2008) Cleavage of normal and pathological forms of alpha-synuclein by neurosin in vitro. Neurosci Lett 436:52–56. doi:10.1016/j.neulet.2008.02.057
Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW (2008) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14:504–506. doi:10.1038/nm1747
Lee HJ, Suk JE, Bae EJ, Lee SJ (2008) Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem Biophys Res Commun 372:423–428. doi:10.1016/j.bbrc.2008.05.045
Lee HJ, Suk JE, Patrick C et al (2010) Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem 285:9262–9272. doi:10.1074/jbc.M109.081125
Li JY, Englund E, Holton JL et al (2008) Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med 14:501–503. doi:10.1038/nm1746
Liddelow S, Barres B (2015) SnapShot: astrocytes in health and disease. Cell 162(1170–1170):e1171. doi:10.1016/j.cell.2015.08.029
Loria F, Diaz-Nido J (2015) Frataxin knockdown in human astrocytes triggers cell death and the release of factors that cause neuronal toxicity. Neurobiol Dis 76:1–12. doi:10.1016/j.nbd.2014.12.017
Luk KC, Covell DJ, Kehm VM et al (2016) Molecular and biological compatibility with host alpha-synuclein influences fibril pathogenicity. Cell Rep 16:3373–3387. doi:10.1016/j.celrep.2016.08.053
Mak SK, McCormack AL, Manning-Bog AB, Cuervo AM, Di Monte DA (2010) Lysosomal degradation of alpha-synuclein in vivo. J Biol Chem 285:13621–13629. doi:10.1074/jbc.M109.074617
Maroteaux L, Campanelli JT, Scheller RH (1988) Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci 8:2804–2815
Masliah E, Rockenstein E, Veinbergs I et al (2000) Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science 287:1265–1269
Moreno-Gonzalez I, Soto C (2011) Misfolded protein aggregates: mechanisms, structures and potential for disease transmission. Semin Cell Dev Biol 22:482–487. doi:10.1016/j.semcdb.2011.04.002
Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L (2007) A global double-fluorescent Cre reporter mouse. Genesis 45:593–605. doi:10.1002/dvg.20335
Pampalakis G, Sykioti VS, Ximerakis M et al (2017) KLK6 proteolysis is implicated in the turnover and uptake of extracellular alpha-synuclein species. Oncotarget 8:14502–14515. doi:10.18632/oncotarget.13264
Peelaerts W, Bousset L, Van der Perren A et al (2015) alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522:340–344. doi:10.1038/nature14547
Pekny M, Nilsson M (2005) Astrocyte activation and reactive gliosis. Glia 50:427–434. doi:10.1002/glia.20207
Pieri L, Chafey P, Le Gall M, Clary G, Melki R, Redeker V (2016) Cellular response of human neuroblastoma cells to alpha-synuclein fibrils, the main constituent of Lewy bodies. Biochem Biophys Acta 1860:8–19. doi:10.1016/j.bbagen.2015.10.007
Recasens A, Dehay B (2014) Alpha-synuclein spreading in Parkinson’s disease. Front Neuroanat 8:159. doi:10.3389/fnana.2014.00159
Rey NL, Wesson DW, Brundin P (2016) The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol Dis. doi:10.1016/j.nbd.2016.12.013
Reyes JF, Rey NL, Bousset L, Melki R, Brundin P, Angot E (2014) Alpha-synuclein transfers from neurons to oligodendrocytes. Glia 62:387–398. doi:10.1002/glia.22611
Ridet JL, Sarkis C, Serguera C, Zennou V, Charneau P, Mallet J (2003) Transplantation of human adult astrocytes: efficiency and safety requirements for an autologous gene therapy. J Neurosci Res 72:704–708. doi:10.1002/jnr.10617
Sacino AN, Brooks MM, Chakrabarty P et al (2017) Proteolysis of alpha-synuclein fibrils in the lysosomal pathway limits induction of inclusion pathology. J Neurochem 140:662–678. doi:10.1111/jnc.13743
Sacino AN, Thomas MA, Ceballos-Diaz C et al (2013) Conformational templating of alpha-synuclein aggregates in neuronal-glial cultures. Mol Neurodegener 8:17. doi:10.1186/1750-1326-8-17
Singleton AB, Farrer M, Johnson J et al (2003) alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302:841. doi:10.1126/science.1090278
Taguchi K, Watanabe Y, Tsujimura A et al (2014) Differential expression of alpha-synuclein in hippocampal neurons. PLoS One 9:e89327. doi:10.1371/journal.pone.0089327
Tatebe H, Watanabe Y, Kasai T et al (2010) Extracellular neurosin degrades alpha-synuclein in cultured cells. Neurosci Res 67:341–346. doi:10.1016/j.neures.2010.04.008
Van der Perren A, Toelen J, Casteels C et al (2015) Longitudinal follow-up and characterization of a robust rat model for Parkinson’s disease based on overexpression of alpha-synuclein with adeno-associated viral vectors. Neurobiol Aging 36:1543–1558. doi:10.1016/j.neurobiolaging.2014.11.015
Volpicelli-Daley LA, Gamble KL, Schultheiss CE, Riddle DM, West AB, Lee VM (2014) Formation of alpha-synuclein Lewy neurite-like aggregates in axons impedes the transport of distinct endosomes. Mol Biol Cell 25:4010–4023. doi:10.1091/mbc.E14-02-0741
Wong YC, Krainc D (2016) Lysosomal trafficking defects link Parkinson’s disease with Gaucher’s disease. Mov Disord 31:1610–1618. doi:10.1002/mds.26802
Zamanian JL, Xu L, Foo LC et al (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32:6391–6410. doi:10.1523/JNEUROSCI.6221-11.2012
Acknowledgements
The authors thank all lab members for discussion and Seng Zhu for assistance in image analysis. We also thank Tracy Bellande for expert technical assistance. We gratefully acknowledge the Imagopole–Citech of Institut Pasteur (Paris), as well as the France–BioImaging infrastructure network supported by the Agence Nationale de la Recherche (ANR-10–INSB–04; Investments for the Future), the Région Ile-de-France (program Domaine d’Intérêt Majeur-Malinf) for the use of the Zeiss LSM 780 Elyra PS1 microscope, and IMAGIF facility for access to Electron Microscopes. We are also grateful for the financial support of Institut Pasteur (Paris). This work was also supported by the Agence Nationale de la Recherche (ANR 16 CE 16 0019 01 NEUROTUNN) and the EC Joint Programme on Neurodegenerative Diseases (JPND-NeuTARGETs-ANR-14-JPCD-0002-02) to CZ and RM; by Equipe FRM (Fondation pour la Recherche Médicale) 2014 (DEQ 20140329557) and by a France Parkinson Grant to CZ; and by the Centre National de la Recherche Scientifique, France Parkinson (Contract 113344), Equipe FRM (Fondation pour la Recherche Médicale) 2016 (DEQ 20160334896), The Fondation de France (Contract 2015-00060936), the Fondation Simone et Cino Del Duca of the Institut de France, and a “Coup d’Elan à la Recherche Française” award from Fondation Bettencourt-Schueller to RM. FL is recipient of a Marie Skłodowska-Curie fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Loria, F., Vargas, J.Y., Bousset, L. et al. α-Synuclein transfer between neurons and astrocytes indicates that astrocytes play a role in degradation rather than in spreading. Acta Neuropathol 134, 789–808 (2017). https://doi.org/10.1007/s00401-017-1746-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-017-1746-2