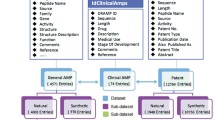
Abstract
We describe computational approaches for identifying promising lead candidates for the development of peptide antibiotics, in the context of quantitative structure–activity relationships (QSAR) studies for this type of molecule. A first approach deals with predicting the selectivity properties of generated antimicrobial peptide sequences in terms of measured therapeutic indices (TI) for known antimicrobial peptides (AMPs). Based on a training set of anuran AMPs, the concept of sequence moments was used to construct algorithms that could predict TIs for a second test set of natural AMPs and could also predict the effect of point mutations on TI values. This approach was then used to design peptide antibiotics (adepantins) not homologous to known natural or synthetic AMPs. In a second approach, many novel putative AMPs were identified from DNA sequences in EST databases, using the observation that, as a rule, specific subclasses of highly conserved signal peptides are associated exclusively with AMPs. Both anuran and teleost sequences were used to elucidate this observation and its implications. The predicted therapeutic indices of identified sequences could then be used to identify new types of selective putative AMPs for future experimental verification.


Similar content being viewed by others
References
Bechinger B, Zasloff M, Opella SJ (1998) Structure and dynamic of the antibiotic peptide PGLa in membranes by solution and solid-state nuclear magnetic resonance technology. Biophys J 74:981–987
Bessalle R, Haas H, Goria A, Shalit I, Fridkin M (1992) Augmentation of the antibacterial activity of magainin by positive-charge chain extension. Antimicrob Agents Chemother 36:313–317
Bhonsle JB, Venugopal D, Huddler DP, Magill AJ, Hicks RP (2007) Application of 3D-QSAR for identification of descriptors defining bioactivity of antimicrobial peptides. J Med Chem 50:6545–6553
Bolintineanu D, Hazrati E, Davis HT, Lehrer RI, Kaznessis YN (2010) Antimicrobial mechanism of pore-forming protegrin peptides: 100 pores to kill E. coli. Peptides 31:1–8
Boman HG, Wade D, Boman IA, Wihlint B, Merrifield RB (1989) Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett 259:103–106
Bommarius B, Kalman D (2009) Antimicrobial and host defense peptides for therapeutic use against multidrug-resistant pathogens: new hope on the horizon. IDrugs 12:376–380
Bowdish DME, Davidson DJ, Monisha G, Scott MG, Hancock REW (2005) Immunomodulatory activities of small host defense peptides. Antimicrob Agents Chemother 49:1727–1732
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria. Nat Rev Microbiol 3:238–250
Castro MS, Cilli EM, Fontes W (2006) Combinatorial synthesis and directed evolution applied to the production of α-helix forming antimicrobial peptides analogues. Curr Protein Pept Sci 7:473–478
Chekmenov EY, Vollmar BS, Cottem M (2010) Can antimicrobial peptides scavenge around a cell in less than a second. Biochim Biophys Acta 1798:228–234
Chen F-Y, Lee M-T, Huang HW (2003) Evidence for membrane thinning effect as the mechanism for peptide-induced pore formation. Biophys J 84:3751–3758
Chen Y, Vasil AI, Rehaume L, Mant CT, Burns JL, Vasil ML, Hancock REW, Hodges RS (2006) Comparison of biophysical and biologic properties of α-helical enantiomeric antimicrobial peptides. Chem Biol Drug Des 67:162–173
Chen LF, Chopra T, Kave KS (2009) Pathogens resistant to antimicrobial agents. Infect Dis Clin North Am 23:817–845
Conlon JM, Sonnevend A, Davidson C, Smith DD, Nielsen PF (2004) The ascaphins: a family of antimicrobial peptides from the skin secretions of the most primitive extant frog, Ascaphus truei. Biochem Biophys Res Commun 320:170–175
Conlon JM, Galadari S, Raza H, Condamine E (2008) Design of potent non-toxic antimicrobial agents based upon the naturally occurring frog skin peptides, ascaphin 8 and peptide XT-7. Chem Biol Drug Des 72:58–64
Conlon JM, Kolodziejek J, Nowotny N (2009) Antimicrobial peptides from the skins of North American frogs. Biochim Biophys Acta 1788:1556–1563
Conticello SG, Gilad Y, Avidan N, Ben-Asher E, Levy Z, Fainzilber M (2001) Mechanisms for evolving hypervariability: the case of conopeptides. Mol Biol Evol 18:120–131
Cuthbertson JM, Doyle DA, Sansom MSP (2005) Transmembrane helix prediction: a comparative evaluation and analysis. Protein Eng Des Select 18:295–308
Dathe M, Nikolenko H, Meyer J, Beyermann M, Bienert M (2001) Optimization of the antimicrobial activity of magainin peptides by modification of charge. FEBS Lett 501:146–150
Desbois AP, Gemmell CG, Coote PJ (2010) In vivo efficacy of the antimicrobial peptide ranalexin in combination with the endopeptidase lysostaphin against wound and systemic meticillin-resistant Staphylococcus aureus (MRSA) infections. Int J Antimicrob Agents 35:559–565
Eisenberg D, Weiss RM, Terwilliger CT, Wilcox W (1982) Hydrophobic moments and protein structure. Faraday Symp Chem Soc 17:109–120
El Amri C, Nicolas P (2008) Plasticins: membrane-damaging peptides with “chameleon-like” properties. Cell Mol Life Sci 65:895–909
El Amri C, Bruston F, Joanne P, Lacombe C, Nicolas P (2007) Intrinsic flexibility and structural adaptability of plasticins membrane-damaging peptides as a strategy for functional versatility. Eur Biophys J 36:901–909
Epand RF, Maloy WL, Ramamoorthy A, Epand RM (2010) Probing the „charge cluster mechanism” in amphipathic helical cationic antimicrobial peptides. Biochemistry 49:4076–4084
Fernandez DI, Gehman JD, Separovic F (2009) Membrane interactions of antimicrobial peptides from Australian frogs. Biochim Biophys Acta 1788:1630–1638
Ferre R, Melo MN, Correia AD, Feliu L, Bardají E, Planas M, Castanho M (2009) Synergistic effects of the membrane actions of cecropin-melittin antimicrobial hybrid peptide BP100. Biophys J 96:1815–1827
Fjell CG, Hancock REW, Cherkasov A (2007) AMPer: a database and an automated discovery tool for antimicrobial peptides. Bioinformatics 23:1148–1155
Fjell CD, Jenssen H, Hilpert K, Cheung WA, Panté N, Hancock REW, Cherkasov A (2009) Identification of novel antibacterial peptides by chemoinformatics and machine learning. J Med Chem 52:2006–2015
Frecer V, Ho B, Ding JL (2004) De novo design of potent antimicrobial peptides. Antimicrob Agents Chemother 48:3349–3357
Fukuoka S, Howe J, Andrä J, Gutsmann T, Rössle M, Brandenburg K (2008) Physico-chemical and biophysical study of the interaction of hexa- and heptaacyl lipid A from Erwinia carotovora with magainin 2-derived antimicrobial peptides. Biochim Biophys Acta 1778:2051–2057
Giangaspero A, Sandri L, Tossi A (2001) Amphipathic α-helical antimicrobial peptides. A systematic study of the effects of structural and physical properties on biological activity. Eur J Biochem 268:5589–5600
Gibson BW, Tang D, Mandrell R, Kelly M, Spindel ER (1991) Bombinin-like peptides with antimicrobial activity from skin secretions of the Asian toad, Bombina orientalis. J Biol Chem 266:23103–23111
Glukhov E, Stark M, Burrows LL, Deber CM (2005) Basis for selectivity of cationic antimicrobial peptides for bacterial versus mammalian membranes. J Biol Chem 280:33960–33967
Gottler LM, Ramamoorthy A (2009) Structure, membrane orientation, mechanism, and function of pexiganan—A highly potent antimicrobial peptide designed from magainin. Biochim Biophys Acta 1788:1680–1686
Grage SL, Afonin S, Urlich AS (2010) Dynamic transitions of membrane-active peptides. Methods Mol Biol 618:183–207
Guy HR (1985) Amino acid side-chain partition energies and distribution of residues in soluble proteins. Biophys J 47:61–70
Hancock REW (2001) Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect Dis 1:156–164
Hancock REW, Lehrer R (1998) Cationic peptides: a new source of antibiotics. Trends Biotechnol 16:82–88
Hancock REW, Sahl H-G (2006) Antimicrobial and host defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24:1551–1557
Haney EF, Hunter HN, Matsuzaki K, Vogel HJ (2009) Solution NMR studies of amphibian antimicrobial peptides: linking structure to function? Biochim Biophys Acta 1788:1639–1655
Hawrani A, Howe RA, Walsh TR, Dempsey CE (2008) Origin of low mammalian cell toxicity in a class of highly active antimicrobial amphipathic helical peptides. J Biol Chem 283:18636–18645
Hilpert K, Fjell CD, Cherkasov A (2008) Short linear cationic antimicrobial peptides: screening optimizing and prediction. Methods Mol Biol 494:127–159
Huang HW (2000) Action of antimicrobial peptides: two state model. Biochemistry 39:8347–8352
Imura Y, Choda N, Matsuzaki K (2008) Magainin 2 in action: distinct modes of membrane permeabilization in living bacterial and mammalian cells. Biophys J 95:5757–5765
Islam K, Hawser SP (1998) MSI-78 (Magainin Pharmaceuticals). IDrugs 1:605–609
Jang H, Ma B, Woolf TB, Nussinov R (2006) Interaction of protegrin-1 with lipid bilayers: membrane thinning effect. Biophys J 91:2848–2859
Janin J (1979) DeltaG-transfer from buried interior to solvent accessible surface. Nature 277:491–492
Jiang Z, Vasil AI, Hale J, Hancock REW, Vasil ML, Hodges RS (2008) Effects of net charge and the number of positively charged residues on the biological activity of amphipathic α-helical cationic antimicrobial peptides. Biopolymers 90:369–383
Jin Y, Mozsolits H, Hammer J, Zmuda E, Zhu F, Zhang Y, Aguilar MI, Blazyk J (2003) Influence of tryptophan on lipid binding of linear amphipathic cationic antimicrobial peptides. Biochemistry 42:9395–9405
Juretić D (1990) Antimicrobial peptides of the magainin family: membrane depolarization studies on E. coli and cytochrome oxidase liposomes. Stud Biophys 138:79–86
Juretić D, Lučin A (1998) The preference functions method for predicting helical turns with membrane propensity. J Chem Inf Comput Sci 38:575–585
Juretić D, Zoranić L, Zucić D (2002) Basic charge clusters and predictions of membrane protein topology. J Chem Inf Comput Sci 42:620–632
Juretić D, Jerončić A, Zucić D (1999) Sequence analysis of membrane proteins with the web server SPLIT. Croat Chem Acta 72:975–997
Juretić D, Vukičević D, Ilić N, Antcheva N, Tossi A (2009) Computational design of highly selective antimicrobial Peptides. J Chem Inf Model 49:2873–2882
Klein E, Smith DL, Laxminarayan R (2007) Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis 13:1840–1846
Kondejewski LH, Jelokhani-Niaraki M, Farmer SW, Lix B, Kay CM, Sykes BD, Hancock REW, Hodges RS (1999) Dissociation of antimicrobial and hemolytic activities in cyclic peptide diastereomers by systematic alterations in amphipathicity. J Biol Chem 274:13181–13192
Konig E, Bininda-Emonds OR (2011) Evidence for convergent evolution in the antimicrobial peptide system in anuran amphibians. Peptides 32:20–25
Kragol G, Lovas S, Varadi G, Condie BA, Hoffmann R, Otvos L Jr (2001) The antibacterial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding. Biochemistry 40:3016–3026
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
La Rocca P, Biggin PC, Tieleman DP, Sanson MS (1999) Simulation studies of the interaction of antimicrobial peptides and lipid bilayers. Biochim Biophys Acta 1462:185–200
Lai Y, Gallo RL (2009) AMPed immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol 30:131–141
Langham AA, Khandelia H, Schuster B, Waring AJ, Lehrer RI, Kaznessis YN (2008) Correlation between simulated physicochemical properties and hemolycity of protegrin-like antimicrobial peptides: Predicting experimental toxicity. Peptides 29:1085–1093
Lauth X, Shike H, Burns JC, Westerman ME, Ostland VE, Carlberg JM, Van Olst JC, Nizet V, Taylor SW, Shimizu C, Bulet P (2002) Discovery and characterization of two isoforms of moronecidin, a novel antimicrobial peptide from hybrid striped bass. J Biol Chem 277:5030–5039
Lejon T, Stiberg T, Strøm MB, Svendsen JS (2004) Prediction of antibiotic activity and synthesis of new pentadecapeptides based on lactoferricins. J Pept Sci 10:329–335
Li Y-D, Xie Z-Y, Du Y-L, Zhou Z, Mao X-M, Lv L-X, Li Y-Q (2009) The rapid evolution of signal peptides is mainly caused by relaxed selection on non-synonymous and synonymous sites. Gene 436:8–11
Lohner K, Prossnigg F (2009) Biological activity and structural aspects of PGLa interaction with membrane mimetic systems. Biochim Biophys Acta 1788:1656–1666
Maloy WL, Kari UP (1995) Structure-activity studies on magainins and other host defense peptides. Biopolymers 37:105–122
Marr AK, Gooderham WJ, Hancock REW (2006) Antimicrobial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol 6:468–472
Mason AJ, Bechinger B, Kichler A (2007) Rational design of vector and antibiotic peptides using solid-state NMR. Mini-Rev Med Chem 7:491–497
Matsuzaki K (1998) Magainins as paradigm for the mode of action of pore forming polypeptides. Biochim Biophys Acta 1376:391–400
Matsuzaki K (2009) Control of cell selectivity of antimicrobial peptides. Biochim Biophys Acta 1788:1687–1692
Matsuzaki K, Murase O, Fujii N, Miyajima K (1995) Translocation of a channel-forming antimicrobial peptide, magainin 2, across lipid bilayers by forming a pore. Biochemistry 34:6521–6526
Mattute B, Knoop FC, Conlon JM (2000) Kassinatuerin-1: a peptide with broadspectrum antimicrobial activity isolated from the skin of the hyperoliid frog, Kassina senegalensis. Biochem Biophys Res Commun 268:433–436
Melnyk RA, Kim S, Curran AR, Engelman DM, Bowie JU, Deber CM (2004) The affinity of GXXXG motifs in transmembrane helix-helix interactions is modulated by long-range communication. J Biol Chem 279:16591–16597
Mihajlovic M, Lazarides T (2010) Antimicrobial peptides bind more strongly to membrane pores. Biochim Biophys Acta 1798:1494–1502
Nicolas P (2009) Multifunctional host defense peptides: intracellular targeting antimicrobial peptides. FEBS J 276:6483–6496
Nicolas P, El Amri C (2009) The dermaseptin superfamily: a gene-based combinatorial library of antimicrobial peptides. Biochim Biophys Acta 1788:1537–1550
Norrby SR, Nord CE, Finch R (2005) Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancelet Infect Dis 5:115–119
Ostberg N, Kaznessis Y (2005) Protegrin structure–activity relationships: using homology models of synthetic sequences to determine structural characteristics important for activity. Peptides 26:197–206
Pál T, Sonnevend A, Galadari S, Conlon JM (2005) Design of potent, non-toxic antimicrobial agents based upon the structure of the frog skin peptide, pseudin-2. Regul Pept 129:85–91
Pál T, Abraham B, Sonnevend A, Jumaa P, Conlon JM (2006) Brevinin-1BYa: a naturally occurring peptide from frog skin with broad-spectrum antibacterial and antifungal properties. Int J Antimicrob Agents 27:525–529
Pandey BK, Ahmad A, Asthana N, Azmi S, Srivastava RM, Srivastava S, Verma R, Vishwakarma AL, Ghosh JK (2010) Cell-selective lysis by novel analogues of melittin against human red blood cells and Escherichia coli. Biochemistry 49:7920–7929
Pathak N, Salas-Auvert R, Ruche G, Janna M-H, McCarthy D, Harrison RG (1995) Comparison of the effect of hydrophobicity, amphiphilicity, and α-helicity on the activities of antimicrobial peptides. Proteins Struct Funct Genet 22:182–186
Pérez-Payá E, Houghten RA, Blondelle SE (1994) Determination of the secondary structure of selected melittin analogues with different haemolytic activities. Biochem J 299:587–591
Perron GG, Zasloff M, Bell G (2006) Experimental evolution of resistance to an antimicrobial peptide. Proc R Soc B 273:251–256
Peschel A, Sahl HG (2006) The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol 4:529–536
Ramamoorthy A (2009) Beyond NMR spectra of antimicrobial peptides: dynamical images at atomic resolution and functional insights. Solid State Nucl Magn Reson 35:201–207
Rockström J, Steffen W, Noone K, Persson A, Chapin FS 3rd, Lambin EF, Lenton TM, Scheffer M, Folke C, Schellnhuber HJ, Nykvist B, de Wit CA, Hughes T, van der Leeuw S, Rodhe H, Sörlin S, Snyder PK, Costanza R, Svedin U, Falkenmark M, Karlberg L, Corell RW, Fabry VJ, Hansen J, Walker B, Liverman D, Richardson K, Crutzen P, Foley JA (2009) A safe operating space for humanity. Nature 461:472–475
Roelants K, Fry BG, Norman JA, Clynen E, Schoofs L, Bossuvt F (2010) Identical skin toxins by convergent molecular adaptation in frogs. Curr Biol 20:125–130
Rollins-Smith LA (2009) The role of amphibian antimicrobial peptides in protection of amphibians from pathogens linked to global amphibian declines. Biochim Biophys Acta 1788:1593–1599
Schneider D, Engelman DM (2004) Motifs of two small residues can assist but are not sufficient to mediate transmembrane helix interactions. J Mol Biol 343:799–804
Senes A, Gerstein M, Engelman DM (2000) Statistical analysis of amino acid patterns in transmembrane helices: the GxxxG motif occurs frequently and in association with β-branched residues at neighboring positions. J Mol Biol 296:921–936
Sengupta D, Leontiadou H, Mark AE, Marrink SJ (2008) Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim Biophys Acta 1778:2308–2317
Siegal RE (2008) Emerging Gram-negative antibiotic resistance: daunting challenges, declining sensitivities and dire consequences. Respir Care 53:471–479
Simmaco M, Mignogna G, Barra D (1998) Antimicrobial peptides from amphibian skin: what do they tell us? Biopolymers 47:435–450
Simmaco M, Kreil G, Barra D (2009) Bombinins, antimicrobial peptides from Bombina species. Biochim Biophys Acta 1788:1551–1555
Steinberg DA, Hurst MA, Fujii CA, Kung AH, Ho JF, Cheng FC, Loury DJ, Fiddes JC (1997) Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob Agents Chemother 41:1738–1742
Strandberg E, Kanithasen N, Tiltak D, Bürck J, Wadhwani P, Zwernemann O, Urlich AC (2008) Solid-state NMR analysis comparing the designer made antibiotic MSI-103 with its parent peptide PGLa in lipid bilayers. Biochemistry 47:2601–2616
Strandberg E, Esteban-Martín S, Salgado J, Ulrich AS (2009a) Orientation and dynamics of peptides in membranes calculated from 2H-NMR data. Biophys J 96:3223–3232
Strandberg E, Tremouilhac P, Wadhwani P, Urlich AS (2009b) Synergistic transmembrane insertion of the heterodimeric PGLa/magainin 2 complex studied by solid-state NMR. Biochim Biophys Acta 1788:1667–1679
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues AS, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786
Sun X, Chen S, Li S, Yan H, Fan Y, Mi H (2005) Deletion of two C-terminal Gln residues of 12–26-residue fragment of melittin improves its antimicrobial activity. Peptides 26:369–375
Taboureau O (2010) Methods for building quantitative structure-activity relationship (QSAR) descriptors and predictive models for computer-aided design of antimicrobial peptides. Methods Mol Biol 618:77–86
Taboureau O, Olsen OH, Nielsen JD, Raventos D, Mygind PH, Kristensen HH (2006) Design of novispirin antimicrobial peptides by quantitative structure-activity relationship. Chem Biol Drug Des 68:48–57
Tachi T, Epand RF, Epand RM, Matsuzaki K (2002) Position dependent hydrophobicity of the antimicrobial magainin peptide affects the mode of peptide-lipid interactions and selective toxicity. Biochemistry 41:10723–10731
Thomas S, Karnik S, Barai RS, Jayaraman VK, Idicula-Thomas S (2010) CAMP: a useful resource for research on antimicrobial peptides. Nucleic Acids Res 38:D774–D780
Tossi A, Tarantino C, Romeo D (1997) Design of synthetic antimicrobial peptides based on sequence analogy and amphipathicity. Eur J Biochem 250:549–558
Tossi A, Sandri L, Giangaspero A (2000) Amphipathic helical antimicrobial peptides. Biopolymers Peptide Sci 55:4–30
Tossi A, Sandri L, Giangaspero A (2002) New consensus hydrophobicity scale extended to non-proteinogenic amino acids. In: Benedetti E, Pedone C (eds) Peptides 2002, proceedings of the 27th European peptide symposium Sorrento August 31st–September 6th 2002. Edizioni Ziino, Napoli, pp 416–417
Tremouilhac P, Strandberg E, Wadhwani P, Ulrich AS (2006) Conditions affecting the re-alignment of the antimicrobial peptide PGLa in membranes as monitored by solid state 2H-NMR. Biochim Biophys Acta 1758:1330–1342
Vanhoye D, Bruston F, Nicolas P, Amiche M (2003) Antimicrobial peptides from hylid and ranin frogs originated from a 150-million-year-old ancestral precursor with a conserved signal peptide but a hypermutable antimicrobial domain. Eur J Biochem 270:2068–2081
Wade D, Andreu D, Mitchell SAN, Silveira AM, Boman A, Boman HG, Merrifield RB (1992) Antibacterial peptides designed as analogs or hybrids of cecropins and melittin. Int J Pept Protein Res 40:429–436
Walters RFS, De Grado WF (2006) Helix-packing motifs in membrane proteins. Proc Natl Acad Sci USA 103:13658–13663
Wang G, Li X, Wang Z (2009) APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res 37:D933–D937
Woodword N (1998) Glycopeptide-resistant enterococci: a decade of experience. J Med Microbiol 47:849–862
Yang N, Stensen W, Svendsen JV, Rekdal Ø (2002) Enhanced antitumor activity and selectivity of lactoferrin-derived peptides. J Pept Res 60:187–197
Yeaman MR, Yount NY (2003) Mechanism of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55
Yeaman MR, Yount NY (2007) Unifying themes in host defence effector polypeptides. Nat Rev Microbiol 5:727–740
Zanetti M (2004) Cathelicidins, multifunctional peptides of the innate immunity. J Leukocyte Biol 75:39–48
Zasloff M (2002) Antimicrobial peptides of multicellular origin. Nature 415:389–395
Zelezetsky I, Tossi A (2006) Alpha-helical antimicrobial peptides–using a sequence template to guide structure-activity relationship studies. Biochim Biophys Acta 1758:1436–1449
Zhang L, Falla TJ (2010) Potential therapeutic application of host defense peptides. Methods Mol Biol 618:303–327
Acknowledgments
This project was carried out as part of the Italy/Croatia Scientific and Technological Cooperation Programme, Project SV2. The work was supported in part by Croatian Ministry of Science, Education and Sport (Grant Nos. 177-1770495-0476 (D.J.), 098-1770495-2919 (B.L.), 177-0000000-0884 (D.V.) and 037-0000000-2779 (D.V.)), and by a Friuli Venezia Giulia LR26 grant for the R3A2 network project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Membrane-active peptides: 455th WE-Heraeus-Seminar and AMP 2010 Workshop.
Rights and permissions
About this article
Cite this article
Juretić, D., Vukičević, D., Petrov, D. et al. Knowledge-based computational methods for identifying or designing novel, non-homologous antimicrobial peptides. Eur Biophys J 40, 371–385 (2011). https://doi.org/10.1007/s00249-011-0674-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-011-0674-7