Abstract
Objective
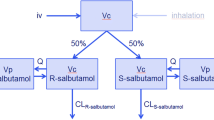
The objective was to determine if the plasma concentrations of salbutamol, obtained during inhalation treatment of infantile acute asthma, are influenced by age range and by the aerosol system used.
Method
A randomized clinical trial was conducted in 46 children (1–5 years of age) with a diagnosis of acute asthma crisis, established in an emergency room pediatric service. Twenty-five children received salbutamol using a pressurized metered-dose inhaler with spacer (50 µg/kg), and 21 children received salbutamol by nebulization (150 µg/kg),three times during a 1-h period. At the end of the treatment, one blood sample was drawn and the plasma was stored for later determination of salbutamol concentration (liquid chromatography). Salbutamol plasma concentrations were compared in two age groups (≤2 years and >2 years of age). The type of device used (pressurized metered-dose inhaler or nebulizer) and the need of hospitalization were also tested. The Mann-Whitney U test was used with the level of significance set at 5% (P < 0.05).
Results
No differences were detected regarding either the aerosol delivery system used or the need for hospitalization in relation to the plasma concentrations of salbutamol. However, higher plasma levels were found in patients >2 years vs patients ≤2 years [median (IQR): 9.40 (6.32–18.22) vs. 4.65 (2.77–10.10) ng/mL], demonstrating a significance difference (P = 0.05).
Conclusion
Salbutamol plasma concentrations were influenced by age group of the patients submitted to inhalation therapy, even with doses adjusted for body weight. After correcting for the differences in the biovailabilities of the delivery systems, the concentrations were independent of the aerosol delivery device used.

Similar content being viewed by others
References
Rubilar L, Rodriguez-Castro JA, Girardi G (2000) Randomized trial of salbutamol via metered-dose inhaler with spacer versus nebulizer for acute wheezing in children less than 2 years of age. Pediatric Pulmonol 29:264–269
Benedito-Fernández J et al (2004) Salbutamol via metered-dose inhaler with spacer versus nebulization for acute treatment of pediatric asthma in the emergency department. Pediatric Emerg Care 20:656–659
Amirav I, Newhouse MT (1997) Metered-dose inhaler accessory devices in acute asthma. Arch Pediatr Adolesc Med 151:876–882
Amantéa SL et al (2002) Controvérsias no manejo farmacológico da asma aguda infantil. J Pediatr (Rio J) 78(Supl.2):S151–S160
Lipworth BJ, Clark DJ (1997) Effects of airway calibre on lung delivery of nebulised salbutamol. Thorax 52:1036–1039
Closa RM, Ceballos JM, Gomez-Papí A et al (1998) Efficacy of bronchodilators administered by nebulizers versus spacer devices in infants with acute wheezing. Pediatric Pulmonol 26:344–348
Isler FA, Newth CJL (1995) Management of acute asthma in children. Baillieres Clin Paediatr 3:341–378
Reinhardt D et al (1984) Age-dependency of alpha and beta-adrenoceptors on thrombocytes and lymphocytes of asthmatic and nonasthmatic children. Eur J Pediatr 142:111–116
Browne GJ et al (1997) Randomized trial of intravenous salbutamol in early management of acute severe asthma in children. Lancet 49:301–305
Global Initiative for Asthma (GINA) (2009) Pocket guide for asthma management and prevention in children 5 years and younger. http://www.ginasthma.org
Rotta ET, Amantéa SL, Froehlich PE, Becker A (2007) Determining salbutamol plasma concentrations after nebulization in pediatric emergency department. J Ped (RioJ) 83(5):481–484
Hutchings MJ, Paull JD, Morgan DJ (1983) Determination of salbutamol in plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr 277:423–426
Penna AC et al (1993) Systemic absorption of salbutamol following nebulizer delivery in acute asthma. Acta Paediatr 82:963–966
Schuh S, Reider MJ, Canny G et al (1990) Nebulized albuterol in acute childhood asthma: comparison of two doses. Pediatrics 86:509–513
Kerem E et al (1993) Efficacy of albuterol administered by nebulizer versus spacer device in children with acute asthma. Pediatr Pharmacol Ther 123:313–317
Cates CJ et al (2006) Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. doi:10.1002/14651858.CD000052.pub2
Deerojanawong J et al (2005) Randomized controlled trial of salbutamol aerosol therapy via metered dose inhaler-spacer vs jet nebulizer in young with children wheezing. Pediatr Pulmonol 39:466–472
Schuh S et al (1999) Comparison of albuterol delivered by a metered dose inhaler with spacer versus a nebulizer in children with mild acute asthma. J Pediatr 135:22–27
Ploin D et al (2000) High-dose albuterol by metered-dose inhaler plus a spacer device versus nebulization in preschool children with recurrent wheezing: a double-blind, randomized equivalence trial. Pediatrics 106:311–331
Delgado A et al (2003) Nebulizers vs metered-dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatric Adolesc Med 157:76–80
Bonnelykke K, Jespersen JJ, Bisgaard H (2007) Age dependent systemic esposure to inhaled salbutamol. Br J Clin Pharmacol 64:241–244
Lipworth B (1996) Pharmacokinetics of inhaled drugs. Br J Clin Pharmacol 42:697–705
Acknowledgments
This work is connected with the Post-Graduation Program in Health Sciences, Fundação Faculdade Federal de Ciências Médicas de Porto Alegre (FFFCMPA), RS, Brazil. The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Specific contribution of each author: Eloni Rotta participated in the collection and analysis of the data and wrote the initial manuscript; Sérgio L. Amantéa conceptualized and planned the study, participated in the analysis of the data and edited the final draft of the article; Adriana Becker participated in the collection, analysis and writing of the paper at the end of the study; Pedro E. Froehlich participated in the planning, validation of the analytical process, and writing of paper at the end of the study.
Rights and permissions
About this article
Cite this article
Rotta, E.T., Amantéa, S.L., Froehlich, P.E. et al. Plasma concentrations of salbutamol in the treatment of acute asthma in a pediatric emergency. Could age be a parameter of influence?. Eur J Clin Pharmacol 66, 605–610 (2010). https://doi.org/10.1007/s00228-010-0787-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-010-0787-4



