Abstract
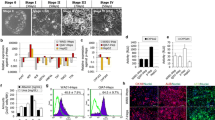
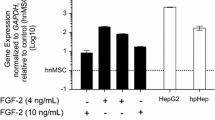
Human-induced pluripotent stem cell-derived hepatocytes (hiPSC-Hep) hold great potential as an unlimited cell source for toxicity testing in drug discovery research. However, little is known about mechanisms of compound toxicity in hiPSC-Hep. In this study, modified mRNA was used to reprogram foreskin fibroblasts into hiPSC that were differentiated into hiPSC-Hep. The hiPSC-Hep expressed characteristic hepatic proteins and exhibited cytochrome P450 (CYP) enzyme activities. Next, the hiPSC-Hep, primary cryopreserved human hepatocytes (cryo-hHep) and the hepatic cell lines HepaRG and Huh7 were treated with staurosporine and acetaminophen, and the toxic responses were compared. In addition, the expression of genes regulating and executing apoptosis was analyzed in the different cell types. Staurosporine, an inducer of apoptosis, decreased ATP levels and activated caspases 3 and 7 in all cell types, but to less extent in Huh7. Furthermore, a hierarchical clustering and a principal component analysis (PCA) of the expression of apoptosis-associated genes separated cryo-hHep from the other cell types, while an enrichment analysis of apoptotic pathways identified hiPSC-Hep as more similar to cryo-hHep than the hepatic cell lines. Finally, acetaminophen induced apoptosis in hiPSC-Hep, HepaRG and Huh7, while the compound initiated a direct necrotic response in cryo-hHep. Our results indicate that for studying compounds initiating apoptosis directly hiPSC-Hep may be a good alternative to cryo-hHep. Furthermore, for compounds with more complex mechanisms of toxicity involving metabolic activation, such as acetaminophen, our data suggest that the cause of cell death depends on a balance between factors controlling death signals and the drug-metabolizing capacity.





Similar content being viewed by others
Abbreviations
- hiPSC:
-
Human-induced pluripotent stem cells
- hiPSC-Hep:
-
Human-induced pluripotent stem cell-derived hepatocytes
- CYP:
-
Cytochrome P450
- cryo-hHep:
-
Cryopreserved human hepatocytes
- PCA:
-
Principal component analysis
- NAPQI:
-
N-acetyl-p-benzoquinone imine
- PAS:
-
Periodic acid schiff
- LC/MS:
-
Liquid chromatography/mass spectrometry
- qPCR:
-
Quantitative real-time PCR
- Ct:
-
Cycle of threshold
- GSH:
-
Glutathione
- RAM:
-
LC-/MS-radiochemical activity monitoring
- HNF4α:
-
Hepatocyte nuclear factor 4 alpha
- CK18:
-
Cytokeratin 18
- AAT:
-
Alpha-1 antitrypsin
- ALB:
-
Albumin
- PXR:
-
Pregnane X receptor
- AFP:
-
Alpha-fetoprotein
References
Antherieu S, Chesne C, Li R, Guguen-Guillouzo C, Guillouzo A (2012) Optimization of the HepaRG cell model for drug metabolism and toxicity studies. Toxicol In Vitro 26(8):1278–1285
Antoine DJ, Dear JW, Lewis PS, Platt V, Coyle J, Masson M, Thanacoody RH, Gray AJ, Webb DJ, Moggs JG, Bateman DN, Goldring CE, Park BK (2013) Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology 58(2):777–787
Dahlin DC, Miwa GT, Lu AY, Nelson SD (1984) N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci USA 81(5):1327–1331
Davidson DG, Eastham WN (1966) Acute liver necrosis following overdose of paracetamol. BMJ 2(5512):497–499
Feng G, Kaplowitz N (2002) Mechanism of staurosporine-induced apoptosis in murine hepatocytes. Am J Physiol Gastrointest Liver Physiol 282(5):G825–G834
Gerets HH, Tilmant K, Gerin B, Chanteux H, Depelchin BO, Dhalluin S, Atienzar FA (2012) Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol Toxicol 28(2):69–87
Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, Cannie I, Guyomard C, Lucas J, Trepo C, Guguen-Guillouzo C (2002) Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci USA 99(24):15655–15660
Guo L, Dial S, Shi L, Branham W, Liu J, Fang JL, Green B, Deng H, Kaput J, Ning B (2011) Similarities and differences in the expression of drug-metabolizing enzymes between human hepatic cell lines and primary human hepatocytes. Drug Metab Dispos 39(3):528–538
Hay DC, Zhao D, Fletcher J, Hewitt ZA, McLean D, Urruticoechea-Uriguen A, Black JR, Elcombe C, Ross JA, Wolf R, Cui W (2008) Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells 26(4):894–902
Hinson JA, Roberts DW, James LP (2010) Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol 196:369–405
Kanebratt KP, Andersson TB (2008a) Evaluation of HepaRG cells as an in vitro model for human drug metabolism studies. Drug Metab Dispos 36(7):1444–1452
Kanebratt KP, Andersson TB (2008b) HepaRG cells as an in vitro model for evaluation of cytochrome P450 induction in humans. Drug Metab Dispos 36(1):137–145
Kia R, Sison RL, Heslop J, Kitteringham NR, Hanley N, Mills JS, Park BK, Goldring CE (2013) Stem cell-derived hepatocytes as a predictive model for drug-induced liver injury: are we there yet? Br J Clin Pharmacol 75(4):885–896
Kola I, Landis J (2004) Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov 3(8):711–715
Laine JE, Auriola S, Pasanen M, Juvonen RO (2009) Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica 39(1):11–21
Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H (1999) Inhibition of fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol Appl Pharmacol 156(3):179–186
Lin J, Schyschka L, Muhl-Benninghaus R, Neumann J, Hao L, Nussler N, Dooley S, Liu L, Stöckle U, Nussler AK, Ehnert S (2012) Comparative analysis of phase I and II enzyme activities in 5 hepatic cell lines identifies huh-7 and HCC-T cells with the highest potential to study drug metabolism. Arch Toxicol 86(1):87–95
Malhi H, Gores GJ, Lemasters JJ (2006) Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology 43(2 Suppl 1):S31–S44
McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H (2011) HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology 53(3):974–982
McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H (2012) The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest 122(4):1574–1583
Nipic D, Pirc A, Banic B, Suput D, Milisav I (2010) Preapoptotic cell stress response of primary hepatocytes. Hepatology 51(6):2140–2151
Persson M, Loye AF, Mow T, Hornberg JJ (2013) A high content screening assay to predict human drug-induced liver injury during drug discovery. J Pharmacol Toxicol Methods 68(3):302–313
Pierce RH, Franklin CC, Campbell JS, Tonge RP, Chen W, Fausto N, Nelson SD, Bruschi SA (2002) Cell culture model for acetaminophen-induced hepatocyte death in vivo. Biochem Pharmacol 64(3):413–424
Possamai LA, McPhail MJ, Quaglia A, Zingarelli V, Abeles RD, Tidswell R, Puthucheary Z, Rawal J, Karvellas CJ, Leslie EM, Hughes RD, Ma Y, Jassem W, Shawcross DL, Bernal W, Dharwan A, Heaton ND, Thursz M, Wendon JA, Mitry RR, Antoniades CG (2013) Character and temporal evolution of apoptosis in acetaminophen-induced acute liver failure*. Crit Care Med 41(11):2543–2550
Richert L, Liguori MJ, Abadie C, Heyd B, Mantion G, Halkic N, Waring JF (2006) Gene expression in human hepatocytes in suspension after isolation is similar to the liver of origin, is not affected by hepatocyte cold storage and cryopreservation, but is strongly changed after hepatocyte plating. Drug Metab Dispos 34(5):870–879
Schaefer O, Ohtsuki S, Kawakami H, Inoue T, Liehner S, Saito A, Sakamoto A, Ishiguro N, Matsumaru T, Terasaki T, Ebner T (2012) Absolute quantification and differential expression of drug transporters, cytochrome P450 enzymes, and UDP-glucuronosyltransferases in cultured primary human hepatocytes. Drug Metab Dispos 40(1):93–103
Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, Chung CW, Jung YK, Oh BH (2001) An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry 40(4):1117–1123
Sivertsson L, Synnergren J, Jensen J, Bjorquist P, Ingelman-Sundberg M (2013) Hepatic differentiation and maturation of human embryonic stem cells cultured in a perfused three-dimensional bioreactor. Stem Cells Dev 22(4):581–594
Sullivan GJ, Hay DC, Park IH, Fletcher J, Hannoun Z, Payne CM, Dalgetty D, Black JR, Ross JA, Samuel K, Wang G, Daley GQ, Lee JH, Church GM, Forbes SJ, Iredale JP, Wilmut I (2010) Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology 51(1):329–335
Takayama K, Kawabata K, Nagamoto Y, Kishimoto K, Tashiro K, Sakurai F, Tachibana M, Kanda K, Hayakawa T, Furue MK, Mizuguchi H (2013) 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing. Biomaterials 34(7):1781–1789
Ulvestad M, Nordell P, Asplund A, Rehnstrom M, Jacobsson S, Holmgren G, Davidson L, Brolén G, Edsbagge J, Björquist P, Küppers-Munther B, Andersson TB (2013) Drug metabolizing enzyme and transporter protein profiles of hepatocytes derived from human embryonic and induced pluripotent stem cells. Biochem Pharmacol 86(5):691–702
Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ (2010) Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7(5):618–630
Wobus AM, Loser P (2011) Present state and future perspectives of using pluripotent stem cells in toxicology research. Arch Toxicol 85(2):79–117
Yildirimman R, Brolen G, Vilardell M, Eriksson G, Synnergren J, Gmuender H, Kamburov A, Ingelman-Sundberg M, Castell J, Lahoz A, Kleinjans J, van Delft J, Björquist P, Herwig R (2011) Human embryonic stem cell derived hepatocyte-like cells as a tool for in vitro hazard assessment of chemical carcinogenicity. Toxicol Sci 124(2):278–290
Acknowledgments
This study was in part supported by SCR&Tox, which is funded by the European Commission within its seventh framework Programme and Cosmetics Europe, the European Cosmetics Association, as part of the SEURAT (Toward the replacement of in vivo repeated dose system toxicity) Cluster [Contract HEALTH-F5–2010-266573]. We thank Isabelle Jansson, Anette Persson-Kry and Anna Svensson (Discovery Sciences, Reagents and Assay Development, AstraZeneca R&D, Mölndal, Sweden) for contributing with cell culture work and Frank Seeliger (Drug Safety and Metabolism, Pathology Sciences, AstraZeneca R&D, Mölndal, Sweden) for histological evaluations of teratoma.
Conflict of interest
The authors declare no conflict of interest.
Ethical standard
The manuscript does not contain clinical studies or patient data.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sjogren, AK.M., Liljevald, M., Glinghammar, B. et al. Critical differences in toxicity mechanisms in induced pluripotent stem cell-derived hepatocytes, hepatic cell lines and primary hepatocytes. Arch Toxicol 88, 1427–1437 (2014). https://doi.org/10.1007/s00204-014-1265-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-014-1265-z