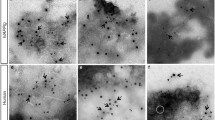
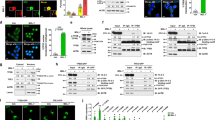
Abstract
BACE2 (β-site APP-cleaving enzyme 2) is a protease expressed in the brain, but also in the pancreas, where it seems to play a physiological role. Amyloidogenic diseases, including Alzheimer’s disease and type 2 diabetes (T2D), share the accumulation of abnormally folded and insoluble proteins that interfere with cell function. In T2D, islet amyloid polypeptide (IAPP) deposits have been shown to be a pathogenic key feature of the disease. The aim of the present study was to investigate the effect of BACE2 modulation on β-cell alterations in a mouse model of T2D induced by IAPP overexpression. Heterozygous mice carrying the human transcript of IAPP (hIAPP-Tg) were used as a model to study the deleterious effects of IAPP upon β-cell function. These animals showed glucose intolerance and impaired insulin secretion. When crossed with BACE2-deficient mice, the animals presented a significant improvement in glucose tolerance accompanied with an enhanced insulin secretion, as compared to hIAPP-Tg mice. BACE2 deficiency also partially reverted gene expression changes observed in islets from hIAPP-Tg mice, including a set of genes related to inflammation. Moreover, homozygous hIAPP mice presented a severe hyperglycemia and a high lethality rate from 8 weeks onwards due to a massive destruction of β-cell mass. This process was significantly reduced when crossed with the BACE2-KO model, improving the survival rate of the animals. Altogether, the absence of BACE2 ameliorates glucose tolerance defects induced by IAPP overexpression in the β-cell and promotes β-cell survival. Thus, targeting BACE2 may represent a promising therapeutic strategy to improve β-cell function in T2D.






Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer disease
- BACE1:
-
β-Site APP-cleaving enzyme 1
- BACE2:
-
β-Site APP-cleaving enzyme 2
- GSIS:
-
Glucose-stimulated insulin secretion
- GTT:
-
Glucose tolerance test
- IAPP:
-
Islet amyloid polypeptide
- PCR:
-
Polymerase chain reaction
- mRNA:
-
Messenger RNA
- T2D:
-
Type 2 diabetes mellitus
References
Saunders A, Kim T-W, Tanzi R (1999) BACE maps to chromosome 11 and a BACE homolog, BACE2, reside in the obligate Down Syndrome region of chromosome 21. Science 286:1255a
Sun X, Wang Y, Qing H et al (2005) Distinct transcriptional regulation and function of the human BACE2 and BACE1 genes. FASEB J 19:739–749. doi:10.1096/fj.04-3426com
Lahiri D, Maloney B, Ge Y (2006) Functional domains of the BACE1 and BACE2 promoters and mechanisms of transcriptional suppression of the BACE2 promoter in normal neuronal cells. J Mol Neurosci 29:65–80. doi:10.1385/JMN/29
Sinha S, Anderson JP, Barbour R et al (1999) Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature 402:537–540. doi:10.1038/990114
Yan R, Bienkowski MJ, Shuck ME et al (1999) Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature 402:533–537. doi:10.1038/990107
Bennett BD, Babu-Khan S, Loeloff R et al (2000) Expression analysis of BACE2 in brain and peripheral tissues. J Biol Chem 275:20647–20651. doi:10.1074/jbc.M002688200
Alcarraz-Vizán G, Casini P, Cadavez L et al (2015) Inhibition of BACE2 counteracts hIAPP-induced insulin secretory defects in pancreatic b-cells. FASEB J 29:95–104. doi:10.1096/fj.14-255489
Vassar R, Bennett BD, Babu-Khan S et al (1999) Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286:735–741. doi:10.1126/science.286.5440.735
Farzan M, Schnitzler CE, Vasilieva N et al (2000) BACE2, a beta -secretase homolog, cleaves at the beta site and within the amyloid-beta region of the amyloid-beta precursor protein. Proc Natl Acad Sci USA 97:9712–9717. doi:10.1073/pnas.160115697
Esterhazy D, Stutzer I, Wang H et al (2011) Bace2 is a b cell-enriched protease that regulates pancreatic b cell function and mass. Cell Metab 14:365–377. doi:10.1016/j.cmet.2011.06.018
Kahn SE, Alessio DAD, Schwartz MW et al (1990) Evidence of cosecretion of islet amyloid polypeptide and insulin by b-cells. Diabetes 39:634–638. doi:10.2337/diabetes.39.5.634
Zhang X-X, Pan Y-H, Huang Y-M (2016) Neuroendocrine hormone amylin in diabetes. World J Diabetes 7:189–197. doi:10.4239/wjd.v7.i9.189
Visa M, Alcarraz-Vizan G, Montane J et al (2015) Islet amyloid polypeptide exerts a novel autocrine action in b-cell signaling and proliferation. FASEB J 29:2970–2979. doi:10.1096/fj.15-270553
Mukherjee A, Morales-Scheihing D, Butler PC, Soto C (2015) Type 2 diabetes as a protein misfolding disease. Trends Mol Med 21:439–449. doi:10.1016/j.molmed.2015.04.005
Cooper GJS, Willis AC, Sim RB, Reid KBM (1987) Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Med Sci Purif 84:8628–8632
Clark A, Charge SB, Badman MK et al (1996) Islet amyloid polypeptide: actions and role in the pathogenesis of diabetes. Biochem Soc Trans 24:594–599
Westermark P, Andersson A, Westermark GT (2011) Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev 91:795–826. doi:10.1152/physrev.00042.2009
Casas S, Gomis R, Gribble F, Altirriba J (2007) Impairment of the ubiquitin-proteasome pathway is a downstream endoplasmic reticulum stress response induced by extracellular human islet amyloid polypeptide and contributes to pancreatic β-cell apoptosis. Diabetes 56:2284–2294. doi:10.2337/db07-0178.Additional
Prentki M, Nolan CJ (2006) Islet b cell failure in type 2 diabetes. J Clin Invest 116:1802–1812. doi:10.1172/JCI29103.1802
Casas S, Casini P, Piquer S et al (2010) BACE2 plays a role in the insulin receptor trafficking in pancreatic β-cells. Am J Physiol Endocrinol Metab 299:E1087–E1095. doi:10.1152/ajpendo.00420.2010
Soty M, Visa M, Soriano S et al (2011) Involvement of ATP-sensitive potassium (K ATP) channels in the loss of beta-cell function induced by human islet amyloid polypeptide. J Biol Chem 286:40857–40866. doi:10.1074/jbc.M111.232801
Janson J, Soeller W, Roche P et al (1996) Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci USA 93:7283–7288
Moreno-Asso A, Castaño C, Grilli A et al (2013) Glucose regulation of a cell cycle gene module is selectively lost in mouse pancreatic islets during ageing. Diabetologia 56:1761–1772. doi:10.1007/s00125-013-2930-0
Karlsson E, Sandler S (2001) Islet amyloid polypeptide promotes beta-cell proliferation in neonatal rat pancreatic islets. Cell 44:1015–1018
Mulder H, Gebre-medhin S, Betsholtz C et al (2000) Islet amyloid polypeptide (amylin)-deficient mice develop a more severe form of alloxan-induced diabetes. Am J Physiol ENdocrinol Metab 278(4):E684–691
Rulifson IC, Cao P, Miao L et al (2016) Identification of human islet amyloid polypeptide as a BACE2 substrate. PLoS One. doi:10.1371/journal.pone.0147254
Boni-Schnetzler M, Thorne J, Parnaud G et al (2008) Increased interleukin (IL)-1b messenger ribonucleic acid expression in b-cells of individuals with type 2 diabetes and regulation of IL-1b in human islets by glucose and autostimulation. J Clin Endocrinol Metab 93:4065–4074. doi:10.1210/jc.2008-0396
Westwell-Roper CY, Chehroudi CA, Denroche HC et al (2014) IL-1 mediates amyloid-associated islet dysfunction and inflammation in human islet amyloid polypeptide transgenic mice. Diabetologia 58:575–585. doi:10.1007/s00125-014-3447-x
Meier DT, Morcos M, Samarasekera T et al (2014) Islet amyloid formation is an important determinant for inducing islet inflammation in high-fat-fed human IAPP transgenic mice. Diabetologia 57:1884–1888. doi:10.1007/s00125-014-3304-y
Westwell-Roper C, Denroche HC, Ehses JA, Verchere CB (2016) Differential activation of innate immune pathways by distinct islet amyloid polypeptide (IAPP) aggregates. J Biol Chem 291:jbc.M115.712455. doi:10.1074/jbc.M115.712455
Westwell-Roper C, Dai DL, Soukhatcheva G et al (2011) IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol 187:2755–2765. doi:10.4049/jimmunol.1002854
Masters SL, Dunne A, Subramanian SL et al (2011) Activation of the Nlrp3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1b in type 2 diabetes. Nat Immunol 11:897–904. doi:10.1038/ni.1935.Activation
Ehses J a, Perren A, Eppler E et al (2007) Increased number of islet associated macrophages in type 2 diabetes. Diabetes. doi:10.2337/db06-1650.AEC
Westwell-Roper CY, Ehses JA, Verchere CB (2014) Resident macrophages mediate islet amyloid polypeptide-induced islet IL-1 b production and b-cell dysfunction. Diabetes 63:1698–1711. doi:10.2337/db13-0863
Larsen C, Faulenbach M, Vaag A, et al (2007) Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 357:302–303. doi:10.1056/NEJMc071324 (author reply 303)
Butler AE, Janson J, Bonner-Weir S et al (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110. doi:10.2337/diabetes.52.9.2304
Mathis D, Vence L, Benoist C (2001) B-cell death during progression to diabetes. Nature 414:792–798. doi:10.1038/414792a
Tonne JM, Sakuma T, Munoz-Gomez M et al (2014) Beta cell regeneration after single-round immunological destruction in a mouse model. Diabetologia 58:313–323. doi:10.1007/s00125-014-3416-4
Ardestani A, Maedler K (2016) MST1: a promising therapeutic target to restore functional beta cell mass in diabetes. Diabetologia 59:1843–1849. doi:10.1007/s00125-016-3892-9
Akpinar P, Kuwajima S, Krützfeldt J, Stoffel M (2005) Tmem27: a cleaved and shed plasma membrane protein that stimulates pancreatic beta cell proliferation. Cell Metab 2:385–397. doi:10.1016/j.cmet.2005.11.001
Fukui K, Yang Q, Cao Y et al (2005) The HNF-1 target Collectrin controls insulin exocytosis by SNARE complex formation. Cell Metab 2:373–384. doi:10.1016/j.cmet.2005.11.003
Stützer I, Selevsek N, Esterházy D et al (2013) Systematic proteomic analysis identifies β-site amyloid precursor protein cleaving enzyme 2 and 1 (BACE2 and BACE1) substrates in pancreatic β-cells. J Biol Chem 288:10536–10547. doi:10.1074/jbc.M112.444703
Hemming ML, Elias JE, Gygi SP, Selkoe DJ (2009) Identification of b-secretase (BACE1) substrates using quantitative proteomics. PLoS One. doi:10.1371/journal.pone.0008477
Hill D, Petrik J, Arany E (1998) Growth factors and the regulation of fetal growth. Diabetes Care 21:B60–B69
Garcia-Ocaña A, Takane KK, Syed MA et al (2000) Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem 275:1226–1232. doi:10.1074/jbc.275.2.1226
Gilbert EJ (2013) 1,3-Oxazines as BACE1 and/or BACE2 inhibitors: a patent evaluation (WO2012156284). Expert Opin Ther Patents 23:1069–1073. doi:10.1517/13543776.2013.818134
Southan C (2013) BACE2 as a new diabetes target: a patent review (2010–2012). Expert Opin Ther Patents 23:649–663. doi:10.1517/13543776.2013.780032
Southan C, Hancock JM (2013) A tale of two drug targets: the evolutionary history of BACE1 and BACE2. Front Genet 4:1–20. doi:10.3389/fgene.2013.00293
Acknowledgements
The authors thank Ms. Kimberly Katte for valuable assistance and comments in the preparation of the manuscript. This work was supported by Sardà Farriol Research Programme and by Grants FIS PI11/00679 and PI14/00447, from the Instituto de Salud Carlos III–Subdirección General de Evaluación y Fomento de la Investigación, Fondo Europeo de Desarrollo Regional (FEDER funds), Unión Europea, Una manera de hacer Europa. It also received support from Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM) and from the Project 2014_SGR_520 of the Department of Universities, Research and Information Society of the Government of Catalonia. JM is a recipient of an IDIBAPS Postdoctoral Fellowship-BIOTRACK, supported by the European Community’s Seventh Framework Programme (ECFP7/2007–2013) under Grant Agreement Number 229673.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Alcarraz-Vizán, G., Castaño, C., Visa, M. et al. BACE2 suppression promotes β-cell survival and function in a model of type 2 diabetes induced by human islet amyloid polypeptide overexpression. Cell. Mol. Life Sci. 74, 2827–2838 (2017). https://doi.org/10.1007/s00018-017-2505-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2505-1