Abstract
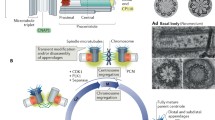
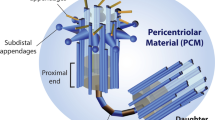
Centrosomes are organelles involved in generating and organizing the interphase microtubule cytoskeleton, mitotic spindles and cilia. At the centrosome core are a pair of centrioles, structures that act as the duplicating elements of this organelle. Centrioles function to recruit and organize pericentriolar material which nucleates microtubules. While centrioles are relatively simple in construction, the mechanics of centriole biogenesis remain an important yet poorly understood process. More mysterious still are the regulatory mechanisms that oversee centriole assembly. The fidelity of centriole duplication is critical as defects in either the assembly or number of centrioles promote aneuploidy, primary microcephaly, birth defects, ciliopathies and tumorigenesis. In addition, some pathogens employ mechanisms to promote centriole overduplication to the detriment of the host cell. This review summarizes our current understanding of this important topic, highlighting the need for further study if new therapeutics are to be developed to treat diseases arising from defects of centrosome duplication.


Similar content being viewed by others
Abbreviations
- APC:
-
Anaphase-promoting complex
- Asl:
-
Asterless protein
- PCM:
-
Pericentriolar material
- Plk4:
-
Polo-like kinase 4
- PP2A:
-
Protein phosphatase 2A
- pre-RC:
-
Pre-replication complex
- ST:
-
Small tumor antigen
- SV40:
-
Simian virus 40
References
Song MH, Miliaras NB, Peel N, O’Connell KF (2008) Centrioles: some self-assembly required. Curr Opin Cell Biol 20:688–693
Nigg EA (2007) Centrosome duplication: of rules and licenses. Trends Cell Biol 17(215):221
Ganem NJ, Godinho SA, Pellman D (2009) A mechanism linking extra centrosomes to chromosomal instability. Nature 460:278–282
Silkworth WT, Nardi IK, Scholl LM, Cimini D (2009) Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis- segregation in cancer cells. PLoS One 4:e6564
Crasta K, Ganem NJ, Dagher R, Lantermann AB, Ivanova EV, Pan Y, Nezi L, Protopopov A, Chowdhury D, Pellman D (2012) DNA breaks and chromosome pulverization from errors in mitosis. Nature 482:53–58
Storchova Z, Pellman D (2004) From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol 5:45–54
Pellman D (2007) Aneuploidy and cancer. Nature 446:38–39
Pihan GA, Wallace J, Zhou Y, Doxsey SJ (2003) Centrosome abnormalities and chromosome instability occur together in pre-invasive carcinomas. Cancer Res 63:1398–1404
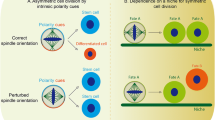
Rusan NM, Peifer M (2007) A role for a novel centrosome cycle in asymmetric cell division. J Cell Biol 177:13–20
Castellanos E, Dominguez P, Gonzalez C (2008) Centrosome dysfunction in Drosophila neural stem cells causes tumors that are not due to genome instability. Curr Biol 18:1209–1214
Basto R, Brunk K, Vinadogrova T, Peel N, Franz A, Khodjakov A, Raff JW (2008) Centrosome amplification can initiate tumorigenesis in flies. Cell 133:1032–1042
Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, Raff JW (2006) Flies without centrioles. Cell 125:1375–1386
Delaval B, Doxsey S (2008) Dwarfism, where pericentrin gains stature. Science 319:732–733
Delaval B, Doxsey SJ (2010) Pericentrin in cellular function and disease. J Cell Biol 188:181–190
Simons M, Walz G (2006) Polycystic kidney disease: cell division without a c(l)ue? Kidney Int 70:854–864
Kochanski RS, Borisy GG (1990) Mode of centriole duplication and distribution. J Cell Biol 110:1599–1605
Riparbelli MG, Callaini G (2003) Drosophila parthenogenesis: a model for de novo centrosome assembly. Dev Biol 260:298–313
Riparbelli MG, Stouthamer R, Dallai R, Callaini G (1998) Microtubule organization during the early development of the parthenogenetic egg of the hymenopteran Muscidifurax uniraptor. Dev Biol 19:589–599
Ferree PM, McDonald K, Fasulo B, Sullivan W (2006) The origin of centrosomes in parthenogenetic hymenopteran insects. Curr Biol 16:801–807
Peel N, Stevens NR, Basto R, Raff JW (2007) Overexpressing centriole replication proteins in vivo induces centriole overduplication and de novo formation. Curr Biol 17:834–843
Khodjakov A, Rieder CL, Sluder G, Cassels G, Sibon O, Wang CL (2002) De novo formation of centrosomes in vertebrate cells arrested during S phase. J Cell Biol 158:1171–1181
Rodrigues-Martins A, Riparbelli M, Callaini G, Glover DM, Bettencourt-Dias M (2007) Revisiting the role of the mother centriole in centriole biogenesis. Science 316:1046–1050
Dirksen ER (1991) Centriole and basal body formation during ciliogenesis revisited. Biol Cell 72:31–38
Kallenbach RJ, Mazia D (1982) Origin and maturation of centrioles in association with the nuclear envelope in hypertonic-stressed sea urchin eggs. Eur J Cell Biol 28:68–76
Anderson RG, Brenner RM (1971) The formation of basal bodies (centrioles) in the Rhesus monkey oviduct. J Cell Biol 50:10–34
Kuriyama R, Borisy GG (1981) Centriole cycle in Chinese hamster ovary cells as determined by whole-mount electron microscopy. J Cell Biol 91:814–821
Robbins E, Jentzsch G, Micali A (1968) The centriole cycle in synchronized HeLa cells. J Cell Biol 36:329–339
Vorobjev IA, Chentsov YuS (1982) Centrioles in the cell cycle, I. Epithelial cells. J Cell Biol 93:938–949
Alvey PL (1985) An investigation of the centriole cycle using 3T3 and CHO cells. J Cell Sci 78:147–162
Pelletier L, O’Toole E, Schwager A, Hyman AA, Muller-Reichert T (2006) Centriole assembly in Caenorhabditis elegans. Nature 444:619–623
Leidel S, Delattre M, Cerutti L, Baumer K, Gonczy P (2005) SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat Cell Biol 7:115–125
Dammerman A, Muller-Reichert T, Pelletier L, Habermann B, Desai A, Oegema K (2004) Centriole assembly requires both centriolar and pericentriolar material proteins. Dev Cell 7:815–829
Nakazawa Y, Hiraki M, Kamiya R, Hirono M (2007) SAS-6 is a cartwheel protein that establishes the 9-fold symmetry of the centriole. Curr Biol 17:2169–2174
van Breugel M, Hirono M, Andreeva A, Yanagisawa HA, Yamaguchi S, Nakazawa Y, Morgner N, Petrovich M, Ebong IO, Robinson CV, Johnson CM, Veprintsev D, Zuber B (2011) Structures of SAS-6 suggest its organization in centrioles. Science 331:1196–1199
Kitagawa D, Vakonakis I, Olieric N, Hilbert M, Keller D, Olieric V, Bortfeld M, Erat MC, Fluckiger I, Gonczy P (2011) Structural basis of the 9-fold symmetry of centrioles. Cell 144:364–375
Hiraki M, Nakazawa Y, Kamiya R, Hirono M (2007) Bld10p constitutes the cartwheel-spoke tip and stabilizes the 9-fold symmetry of the centriole. Curr Biol 17:1778–1783
Delattre M, Leidel S, Wani K, Baumer K, Bamat J, Schnabel H, Feichtinger R, Schnabel R, Gonczy P (2004) Centriolar SAS-5 is required for centrosome duplication in C. elegans. Nat Cell Biol 6:656–664
Stevens NR, Dobbelaere J, Brunk K, Franz A, Raff JW (2010) Drosophila Ana2 is a conserved centriole duplication factor. J Cell Biol 188:313–323
Stevens NR, Roque H, Raff JW (2010) Sas-6 and Ana2 coassemble into tubules to promote centriole duplication and engagement. Dev Cell 19:913–919
Leidel S, Gonczy P (2003) SAS-4 is essential for centrosome duplication in C. elegans and is recruited to daughter centrioles once per cell cycle. Dev Cell 4:431–439
Kirkham M, Muller-Reichert T, Oegema K, Grill S, Hyman AA (2003) SAS-4 is a C. elegans centriolar protein that controls centrosome size. Cell 112:575–587
Kohlmaier G, Loncarek J, Meng X, McEwen BF, Mogensen MM, Spektor A, Dynlacht BD, Khodjakov A, Gonczy P (2009) Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP. Curr Biol 19:1012–1018
Gopalakrishnan J, Mennella V, Blachon S, Zhai B, Smith AH, Megraw TL, Nicastro D, Gygi SP, Agard DA, Avidor-Reiss T (2011) Sas-4 provides a scaffold for cytoplasmic complexes and tethers them in a centrosome. Nat Commun 2:359. doi:10.1038/ncomms1367
Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA (2007) Plk4-induced centriole biogenesis in human cells. Dev Cell 13:190–202
Spektor A, Tsang WY, Khoo D, Dynlacht BD (2007) Cep97 and CP110 suppress a cilia assembly program. Cell 130:678–690
Chen Z, Indjeian VB, McManus M, Wang L, Dynlacht BD (2002) CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev Cell 3:339–350
Schmidt TI, Kleylein-Sohn J, Westendorf J, Le Clech M, Lavoie SB, Stierhof YD, Nigg EA (2009) Control of centriole length by CPAP and CP110. Curr Biol 19:1005–1011
Singla V, Romaguera-Ros M, Garcia-Verdugo JM, Reiter JF (2010) Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev Cell 18:410–424
Kobayashi T, Dynlacht BD (2011) Regulating the transition from centriole to basal body. J Cell Biol 193:435–444
Hannak E, Kirkham M, Hyman AA, Oegema K (2001) Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J Cell Biol 155:1109–1116
Berdnik D, Knoblich JA (2002) Drosophila Aurora-A is required for centrosome maturation and actin-dependent asymmetric protein localization during mitosis. Curr Biol 12:640–647
Lane HA, Nigg EA (1996) Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol 135:1701–1713
Loncarek J, Hergert P, Magidson V, Khodjakov A (2008) Control of daughter centriole formation by the pericentriolar material. Nat Cell Biol 103:22–28
Tsou MF, Wang WJ, George KA, Uryu K, Stearns T, Jallepalli PV (2009) Polo kinase and separase regulate the mitotic licensing of centriole duplication in human cells. Dev Cell 17:344–354
Loncarek J, Hergert P, Khodjakov A (2010) Centriole reduplication during prolonged interphase requires procentriole maturation governed by Plk1. Curr Biol 20:1277–1282
Wang WJ, Soni RK, Uryu K, Tsou MF (2011) The conversion of centrioles to centrosomes: essential coupling of duplication with segregation. J Cell Biol 193:727–739
Wu ZQ, Liu X (2008) Role for Plk1 phosphorylation of Hbo1 in regulation of replication licensing. Proc Natl Acad Sci USA 105:1919–1924
Blow JJ, Dutta A (2005) Preventing re-replication of chromosomal DNA. Nat Rev Mol Cell Biol 6:476–486
DePamphilis ML, Blow JJ, Ghosh S, Saha T, Noguchi K, Vassilev A (2006) Regulating the licensing of DNA replication origins in metazoa. Curr Opin Cell Biol 18:231–239
Machida YJ, Hamlin JL, Dutta A (2005) Right place, right time, and only once: replication initiation in metazoans. Cell 12:313–324
Wong C, Stearns T (2003) Centrosome number is controlled by a centrosome-intrinsic block to reduplication. Nat Cell Biol 5:539–544
Tsou MF, Stearns T (2006) Mechanism limiting centrosome duplication to once per cell cycle. Nature 442:947–951
Hinchcliffe EH, Li C, Thompson EA, Maller JL, Sluder G (1999) Requirement of Cdk2–cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science 283:851–854
Lacey KR, Jackson PK, Stearns T (1999) Cyclin-dependent kinase control of centrosome duplication. Proc Natl Acad Sci USA 96:2817–2822
Matsumoto Y, Hayashi K, Nishida E (1999) Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr Biol 9:429–432
Meraldi P, Lukas J, Fry AM, Bartek J, Nigg EA (1999) Centrosome duplication in mammalian somatic cells requires E2F and Cdk2-cyclin A. Nat Cell Biol 1:88–93
Okuda M, Horn HF, Tarapore P, Tokuyama Y, Smulian AG, Chan PK, Knudsen ES, Hofmann IA, Snyder JD, Bove KE, Fukasawa K (2000) Nucleophosmin/B23 is a target of CDK2-cyclin E in centrosome duplication. Cell 103:127–140
Fisk HA, Winey A (2001) The mouse Mps1p-like kinase regulates centrosome duplication. Cell 106:95–104
Fisk HA, Mattison CP, Winey M (2003) Human Mps1 protein kinase is required for centrosome duplication and normal mitotic progression. Proc Natl Acad Sci USA 100:14875–14880
Kasbek C, Yang CH, Yusof AM, Chapman HM, Winey M, Fisk HA (2007) Preventing the degradation of mps1 at centrosomes is sufficient to cause centrosome reduplication in human cells. Mol Biol Cell 18:4457–4469
Shinmura K, Tarapore P, Tokuyama Y, George KR, Fukasawa K (2005) Characterization of centrosomal association of nucleophosmin/B23 linked to Crm1 activity. FEBS Lett 579:6621–6634
Duensing A, Liu Y, Tseng M, Malumbres M, Barbacid M, Duensing S (2006) Cyclin-dependent kinase 2 is dispensable for normal centrosome duplication but required for oncogene-induced centrosome overduplication. Oncogene 252:943–949
Matsumoto Y, Maller JL (2002) Calcium, calmodulin, and CaMKII requirement for initiation of centrosome duplication in Xenopus egg extracts. Science 29:5499–5502
Hinchcliffe EH, Cassels GO, CL Rieder, Sluder G (1998) The coordination of centrosome reproduction with nuclear events of the cell cycle in the sea urchin zygote. J Cell Biol 140:1417–1426
Tsou MF, Stearns T (2006) Controlling centrosome number: licenses and blocks. Curr Opin Cell Biol 18:74–78
O’Connell BC, Harper JW (2007) Ubiquitin proteasome system (UPS): what can chromatin do for you? Curr Opin Cell Biol 19:206–214
Ferenbach A, Li A, Brito-Martins M, Blow JJ (2005) Functional domains of the Xenopus replication licensing factor CDT. Nucleic Acids Res 33:316–324
Wei Z, Liu C, Wu X, Xu N, Zhou B, Liang C, Zhu G (2010) Characterization and structure determination of the Cdt1 binding domain of human minichromosome maintenance (Mcm) 6. J Biol Chem 285:12469–12473
Nakamura A, Arai H, Fujita N (2009) Centrosomal Aki1 and cohesion function in separase-regulated centriole disengagement. J Cell Biol 187:607–614
Schöckel L, Möckel M, Mayer B, Boos D, Stemmann O (2011) Cleavage of cohesin rings coordinates the separation of centrioles and chromatids. Nat Cell Biol 13:966–972
Balczon R, Bao L, Zimmer WE, Brown K, Zinkowski RP, Brinkley BR (1995) Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J Cell Biol 130:105–115
Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA (2005) The Polo kinase Plk4 functions in centriole duplication. Nat Cell Biol 71:140–146
Duensing A, Liu Y, Perdreau SA, Kleylein-Sohn J, Nigg EA, Duensing S (2007) Centriole overduplication through the concurrent formation of multiple daughter centrioles at single maternal templates. Oncogene 26:6280–6288
Strnad P, Leidel S, Vinogradova T, Euteneuer U, Khodjakov A, Gönczy P (2007) Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev Cell 13:203–213
Korzeniewski N, Zheng L, Cuevas R, Parry J, Chatterjee P, Anderton B, Duensing A, Münger K, Duensing S (2009) Cullin 1 functions as a centrosomal suppressor of centriole multiplication by regulating polo-like kinase 4 protein levels. Cancer Res 69:6668–6675
Cunha-Ferreira I, Rodrigues-Martins A, Bento I, Riparbelli M, Zhang W, Laue E, Callaini G, Glover DM, Bettencourt-Dias M (2009) The SCF/Slimb ubiquitin ligase limits centrosome amplification through degradation of SAK/PLK4. Curr Biol 19:43–49
Rogers GC, Rusan NM, Roberts DM, Peifer M, Rogers SL (2009) The SCF Slimb ubiquitin ligase regulates Plk4/Sak levels to block centriole reduplication. J Cell Biol 184:225–239
Fode C, Motro B, Yousefi S, Heffernan M, Dennis JW (1994) Sak, a murine protein-serine/threonine kinase that is related to the Drosophila polo kinase and involved in cell proliferation. Proc Natl Acad Sci USA 91:6388–6392
Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, Riparbelli M, Lehmann L, Gatt MK, Carmo N, Balloux F, Callaini G, Glover DM (2005) SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol 152:199–207
Brownlee CW, Klebba JE, Buster DW, Rogers GC (2011) The protein phosphatase 2A regulatory subunit twins stabilizes Plk4 to induce centriole amplification. J Cell Biol 195:231–243
O’Connell KF, Caron C, Kopish KR, Hurd DD, Kemphues KJ, Li Y, White JG (2001) The C. elegans zyg-1 gene encodes a regulator of centrosome duplication with distinct maternal and paternal roles in the embryo. Cell 105:547–558
Delattre M, Canard C, Gönczy P (2006) Sequential protein recruitment in C. elegans centriole formation. Curr Biol 16:1844–1849
Kitagawa D, Busso C, Fluckiger I, Gönczy P (2009) Phosphorylation of SAS-6 by ZYG-1 is critical for centriole formation in C. elegans embryos. Dev Cell 17:900–907
Puklowski A, Homsi Y, Keller D, May M, Chauhan S, Kossatz U, Grunwald V, Kubicka S, Pich A, Manns MP et al (2011) The SCF-FBXW5 E3-ubiquitin ligase is regulated by PLK4 and targets HsSAS-6 to control centrosome duplication. Nat Cell Biol 13:1004–1009
Blachon S, Gopalakrishnan J, Omori Y, Polyanovsky A, Church A, Nicastro D, Malicki J, Avidor-Reiss T (2008) Drosophila asterless and vertebrate Cep152 Are orthologs essential for centriole duplication. Genetics 180:2081–2094
Varmark H, Llamazares S, Rebollo E, Lange B, Reina J, Schwartz H, Gonzalez C (2007) Asterless is a centriolar protein required for centrosome function and embryo development in Drosophila. Curr Biol 17:1735–1745
Cizmecioglu O, Arnold M, Bahtz R, Settele F, Ehret L, Haselmann-Weiss U, Antony C, Hoffmann I (2010) Cep152 acts as a scaffold for recruitment of Plk4 and CPAP to the centrosome. J Cell Biol 191:731–739
Dzhindzhev NS, Yu QD, Weiskopf K, Tzolovsky G, Cunha-Ferreira I, Riparbelli M, Rodrigues-Martins A, Bettencourt-Dias M, Callaini G, Glover DM (2010) Asterless is a scaffold for the onset of centriole assembly. Nature 467:714–718
Hatch EM, Kulukian A, Holland AJ, Cleveland DW, Stearns T (2010) Cep152 interacts with Plk4 and is required for centriole duplication. J Cell Biol 19:721–729
Leung GC, Ho CS, Blasutig IM, Murphy JM, Sicheri F (2007) Determination of the Plk4/Sak consensus phosphorylation motif using peptide spot arrays. FEBS Lett 581:77–83
Sillibourne JE, Tack F, Vloemans N, Boeckx A, Thambirajah S, Bonnet P, Ramaekers FC, Bornens M, Grand-Perret T (2010) Autophosphorylation of pololike kinase 4 and its role in centriole duplication. Mol Biol Cell 21:547–561
Holland AJ, Lan W, Niessen S, Hoover H, Cleveland DW (2010) Polo-like kinase 4 kinase activity limits centrosome overduplication by autoregulating its own stability. J Cell Biol 188:191–198
Guderian G, Westendorf J, Uldschmid A, Nigg EA (2010) Plk4 transautophosphorylation regulates centriole number by controlling betaTrCP-mediated degradation. J Cell Sci 123:2163–2169
Leung GC, Hudson JW, Kozarova A, Davidson A, Dennis JW, Sicheri F (2002) The Sak polo-box comprises a structural domain sufficient for mitotic subcellular localization. Nat Struct Biol 9:719–724
Song MH, Liu Y, Anderson DE, Jahng WJ, O’Connell KF (2011) Protein phosphatase 2A-SUR-6/B55 regulates centriole duplication in C. elegans by controlling the levels of centriole assembly factors. Dev Cell 20:563–571
Kitagawa D, Fluckiger I, Polanowska J, Keller D, Reboul J, Gönczy P (2011) PP2A phosphatase acts upon SAS-5 to ensure centriole formation in C. elegans embryos. Dev Cell 20:550–562
Fode C, Binkert C, Dennis JW (1996) Constitutive expression of murine Sak-a suppresses cell growth and induces multinucleation. Mol Cell Biol 16:4665–4672
Chang J, Cizmecioglu O, Hoffmann I, Rhee K (2010) PLK2 phosphorylation is critical for CPAP function in procentriole formation during the centrosome cycle. EMBO J 29:2395–2406
Tang CJ, Lin SY, Hsu WB, Lin YN, Wu CT, Lin YC, Chang CW, Wu KS, Tang TK (2011) The human microcephaly protein STIL interacts with CPAP and is required for procentriole formation. EMBO J 30:4790–4804
Arquint C, Sonnen KF, Stierhof YD, Nigg EA (2012) Cell-cycle-regulated expression of STIL controls centriole number in human cells. J Cell Sci 125:1342–1352
Vulprecht J, David A, Tibelius A, Castiel A, Konotop G, Liu F, Bestvater F, Raab MS, Zentgraf H, Izraeli S, Krämer A (2012) STIL is required for centriole duplication in human cells. J Cell Sci 125:1353–1362
Hagiwara H, Ohwada N, Takata K (2004) Cell biology of normal and abnormal ciliogenesis in the ciliated epithelium. Int Rev Cytol 234:101–141
La Terra S, English CN, Hergert P, McEwen BF, Sluder G, Khodjakov A (2005) The de novo centriole assembly pathway in HeLa cells: cell cycle progression and centriole assembly/maturation. J Cell Biol 168:713–722
Uetake Y, Loncarek J, Nordberg JJ, English CN, La Terra S, Khodjakov A, Sluder G (2007) Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J Cell Biol 176:173–182
Löffler H, Fechter A, Matuszewska M, Saffrich R, Mistrik M, Marhold J, Hornung C, Westermann F, Bartek J, Krämer A (2011) Cep63 recruits Cdk1 to the centrosome: implications for regulation of mitotic entry, centrosome amplification, and genome maintenance. Cancer Res 71:2129–2139
Sir JH, Barr AR, Nicholas AK, Carvalho OP, Khurshid M, Sossick A, Reichelt S, D’Santos C, Woods CG, Gergely F (2011) A primary microcephaly protein complex forms a ring around parental centrioles. Nat Genet 43:1147–1153
Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, Kitagawa M, Nakayama K, Hatakeyama S (2000) Targeted disruption of Skp2 results in accumulation of cyclin E and p27 (Kip1), polyploidy and centrosome overduplication. EMBO J 19:2069–2081
Wojcik EJ, Glover DM, Hays TS (2000) The SCF ubiquitin ligase protein slimb regulates centrosome duplication in Drosophila. Curr Biol 10:1131–1134
Guardavaccaro D, Kudo Y, Boulaire J, Barchi M, Busino L, Donzelli M, Margottin-Goguet F, Jackson PK, Yamasaki L, Pagano M (2003) Control of meiotic and mitotic progression by the F box protein beta-Trcp1 in vivo. Dev Cell 4:799–812
Murphy TD (2003) Drosophila skpA, a component of SCF ubiquitin ligase, regulates centrosome duplication independently of cyclin E accumulation. J Cell Sci 116:2321–2332
Wang W, Budhu A, Forgues M, Wang XW (2005) Temporal and spatial control of nucleophosmin by the Ran-Crm1 complex in centrosome duplication. Nat Cell Biol 7:823–830
Ko MJ, Murata K, Hwang DS, Parvin JD (2006) Inhibition of BRCA1 in breast cell lines causes the centrosome duplication cycle to be disconnected from the cell cycle. Oncogene 25:298–303
Sato K, Hayami R, Wu W et al (2004) Nucleophosmin/B23 is a candidate substrate for the BRCA1-BARD1 ubiquitin ligase. J Biol Chem 279:30919–30922
Starita LM, Machida Y, Sankaran S, Elias JE, Griffin K, Schlegel BP, Gygi SP, Parvin JD (2004) BRCA1-dependent ubiquitination of γ-tubulin regulates centrosome number. Mol Cell Biol 24:8457–8466
Shang Y, Tsao CC, Gorovsky MA (2005) Mutational analyses reveal a novel function of the nucleotide-binding domain of gamma-tubulin in the regulation of basal body biogenesis. J Cell Biol 171:1035–1044
Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF (1996) Abnormal centrosome amplification in the absence of p53. Science 271:1744–1747
Meraldi P, Honda R, Nigg EA (2002) Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J 21:483–492
Borel F, Lohez OD, Lacroix FB, Margolis RL (2002) Multiple centrosomes arise from tetraploidy checkpoint failure and mitotic centrosome clusters in p53 and RB pocket protein-compromised cells. Proc Natl Acad Sci USA 99:9819–9824
D’Assoro AB, Busby R, Suino K, Delva E, Almodovar–Mercado GJ, Johnson H, Folk C, Farrugia DJ, Vasile V, Stivala F, Salisbury JL (2004) Genotoxic stress leads to centrosome amplification in breast cancer cell lines that have an inactive G1/S cell cycle checkpoint. Oncogene 23:4068–4075
Li J, Tan M, Li L, Pamarthy D, Lawrence TS, Sun Y (2005) SAK, a new polo-like kinase, is transcriptionally repressed by p53 and induces apoptosis upon RNAi silencing. Neoplasia 7:312–323
Mandal S, Freije WA, Guptan P, Banerjee U (2010) Metabolic control of G1-S transition: cyclin E degradation by p53-induced activation of the ubiquitin-proteasome system. J Cell Biol 188:473–479
Hemerly AS, Prasanth SG, Siddiqui K, Stillman B (2009) Orc1 controls centriole and centrosome copy number in human cells. Science 32:3789–3793
Wu J, Cho HP, Rhee DB, Johnson DK, Dunlap J, Liu Y, Wang Y (2008) Cdc14B depletion leads to centriole amplification, and its overexpression prevents unscheduled centriole duplication. J Cell Biol 181:475–483
Lu F, Lan R, Zhang H, Jiang Q, Zhang C (2008) Geminin is partially localized to centrosome and plays a role in proper centrosome duplication. Biol Cell 101:273–285
Tachibana KE, Gonzalez MA, Guarguaglini G, Nigg EA, Laskey RA (2005) Depletion of licensing inhibitor geminin causes centrosome overduplication and mitotic defects. EMBO Rep 6:1052–1057
Vidwans SJ, Wong ML, O’Farrell PH (2003) Anomalous centriole configurations are detected in Drosophila wing disc cells upon Cdk1 inactivation. J Cell Sci 116:137–143
Saunders W (2005) Centrosomal amplification and spindle multipolarity in cancer cells. Semin Cancer Biol 15:25–32
Cho US, Morrone S, Sablina AA, Arroyo JD, Hahn WC, Xu W (2007) Structural basis of PP2A inhibition by small t antigen. PLoS Biol 5:e202
Arroyo JD, Hahn WC (2005) Involvement of PP2A in viral and cellular transformation. Oncogene 24:7746–7755
Chen Y, Xu Y, Bao Q, Xing Y, Li Z, Lin Z, Stock JB, Jeffrey PD, Shi Y (2007) Structural and biochemical insights into the regulation of protein phosphatase 2A by small t antigen of SV40. Nat Struct Mol Biol 14:527–534
Kotadia S, Kao LR, Comerford SA, Jones RT, Hammer RE, Megraw TL (2008) PP2A-dependent disruption of centrosome replication and cytoskeleton organization in Drosophila by SV40 small tumor antigen. Oncogene 27:6334–6346
Johnson KA, Tan M, Sütterlin C (2009) Centrosome abnormalities during a Chlamydia trachomatis infection are caused by dysregulation of the normal duplication pathway. Cell Microbiol 11:1064–1073
Ingemarsdotter C, Keller D, Beard P (2010) The DNA damage response to non-replicating adeno-associated virus: centriole overduplication and mitotic catastrophe independent of the spindle checkpoint. Virology 400:271–286
Duensing S, Lee LY, Duensing A, Basile J, Piboonniyom S, Gonzalez S, Crum CP, Munger K (2000) The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci USA 97:10002–10007
Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM (1990) The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129–1136
Tabakin-Fix Y, Azran I, Schavinky-Khrapunsky Y, Levy O, Aboud M (2006) Functional inactivation of p53 by human T-cell leukemia virus type 1 Tax protein: mechanisms and clinical implications. Carcinogenesis 27:673–681
Nitta T, Kanai M, Sugihara E, Tanaka M, Sun B, Nagasawa T, Sonoda S, Saya H, Miwa M (2006) Centrosome amplification in adult T-cell leukemia and human T-cell leukemia virus type 1 Tax-induced human T cells. Cancer Sci 97:836–841
Huo TI, Wang XW, Forgues M, Wu CG, Spillare EA, Giannini C, Brechot C, Harris CC (2001) Hepatitis B virus X mutants derived from human hepatocellular carcinoma retain the ability to abrogate p53-induced apoptosis. Oncogene 20:3620–3628
Wang XW, Forrester K, Yeh H, Feitelson MA, Gu JR, Harris CC (1994) Hepatitis B virus X protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ERCC3. Proc Natl Acad Sci USA 91:2230–2234
Yun C, Cho H, Kim SJ, Lee JH, Park SY, Chan GK, Cho H (2004) Mitotic aberration coupled with centrosome amplification is induced by hepatitis B virus X oncoprotein via the Ras-mitogen-activated protein/extracellular signal-regulated kinase-mitogen-activated protein pathway. Mol Cancer Res 2:159–169
Mantel C, Braun SE, Reid S, Henegariu O, Liu L, Hangoc G, Broxmeyer HE (1999) p21(cip-1/waf-1) deficiency causes deformed nuclear architecture, centriole overduplication, polyploidy, and relaxed microtubule damage checkpoints in human hematopoietic cells. Blood 93:1390–1398
Korzeniewski N, Treat B, Duensing S (2011) The HPV-16 E7 oncoprotein induces centriole multiplication through deregulation of Polo-like kinase 4 expression. Mol Cancer 10:61
Pim D, Massimi P, Dilworth SM, Banks L (2005) Activation of the protein kinase B pathway by the HPV-16 E7 oncoprotein occurs through a mechanism involving interaction with PP2A. Oncogene 24:7830–7838
De Luca A, Mangiacasale R, Severino A, Malquori L, Baldi A, Palena A, Mileo AM, Lavia P, Paggi MG (2003) E1A deregulates the centrosome cycle in a Ran GTPase-dependent manner. Cancer Res 63:1430–1437
Acknowledgments
We would like to thank Daniel W. Buster for critically reading and editing the manuscript and Rachel D. Brownlee for help with the illustrations. We are grateful for support from the NSF IGERT Training Fellowship to C.W.B., the National Cancer Institute P30 CA23074, and the American Cancer Society IRG 74-001-31, the GI SPORE (NCI/NIH P50 CA95060).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brownlee, C.W., Rogers, G.C. Show me your license, please: deregulation of centriole duplication mechanisms that promote amplification. Cell. Mol. Life Sci. 70, 1021–1034 (2013). https://doi.org/10.1007/s00018-012-1102-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1102-6