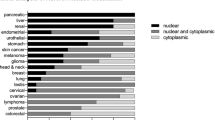
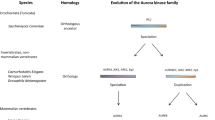
Abstract
Temporally and spatially controlled activation of the Aurora A kinase (AURKA) regulates centrosome maturation, entry into mitosis, formation and function of the bipolar spindle, and cytokinesis. Genetic amplification and mRNA and protein overexpression of Aurora A are common in many types of solid tumor, and associated with aneuploidy, supernumerary centrosomes, defective mitotic spindles, and resistance to apoptosis. These properties have led Aurora A to be considered a high-value target for development of cancer therapeutics, with multiple agents currently in early-phase clinical trials. More recently, identification of additional, non-mitotic functions and means of activation of Aurora A during interphase neurite elongation and ciliary resorption have significantly expanded our understanding of its function, and may offer insights into the clinical performance of Aurora A inhibitors. Here we review the mitotic and non-mitotic functions of Aurora A, discuss Aurora A regulation in the context of protein structural information, and evaluate progress in understanding and inhibiting Aurora A in cancer.






Similar content being viewed by others
References
Chan CS, Botstein D (1993) Isolation and characterization of chromosome-gain and increase-in-ploidy mutants in yeast. Genetics 135:677–691
Paris J, Philippe M (1990) Poly(A) metabolism and polysomal recruitment of maternal mRNAs during early Xenopus development. Dev Biol 140:221–224
Andresson T, Ruderman JV (1998) The kinase Eg2 is a component of the Xenopus oocyte progesterone-activated signaling pathway. EMBO J 17:5627–5637
Roghi C, Giet R, Uzbekov R, Morin N, Chartrain I, Le Guellec R, Couturier A, Doree M, Philippe M, Prigent C (1998) The Xenopus protein kinase pEg2 associates with the centrosome in a cell cycle-dependent manner, binds to the spindle microtubules and is involved in bipolar mitotic spindle assembly. J Cell Sci 111(Pt 5):557–572
Glover DM, Leibowitz MH, McLean DA, Parry H (1995) Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell 81:95–105
Wirtz-Peitz F, Nishimura T, Knoblich JA (2008) Linking cell cycle to asymmetric division: aurora-A phosphorylates the Par complex to regulate Numb localization. Cell 135:161–173
Giet R, McLean D, Descamps S, Lee MJ, Raff JW, Prigent C, Glover DM (2002) Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J Cell Biol 156:437–451
Lee CY, Andersen RO, Cabernard C, Manning L, Tran KD, Lanskey MJ, Bashirullah A, Doe CQ (2006) Drosophila Aurora-A kinase inhibits neuroblast self-renewal by regulating aPKC/Numb cortical polarity and spindle orientation. Gene Dev 20:3464–3474
Wang H, Somers GW, Bashirullah A, Heberlein U, Yu F, Chia W (2006) Aurora-A acts as a tumor suppressor and regulates self-renewal of Drosophila neuroblasts. Gene Dev 20:3453–3463
Vader G, Lens SM (2008) The Aurora kinase family in cell division and cancer. Biochim Biophys Acta 1786:60–72
Meraldi P, Honda R, Nigg EA (2002) Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J 21:483–492
Anand S, Penrhyn-Lowe S, Venkitaraman AR (2003) AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell 3:51–62
Zhang D, Hirota T, Marumoto T, Shimizu M, Kunitoku N, Sasayama T, Arima Y, Feng L, Suzuki M, Takeya M, Saya H (2004) Cre-loxP-controlled periodic Aurora-A overexpression induces mitotic abnormalities and hyperplasia in mammary glands of mouse models. Oncogene 23:8720–8730
Tatsuka M, Katayama H, Ota T, Tanaka T, Odashima S, Suzuki F, Terada Y (1998) Multinuclearity and increased ploidy caused by overexpression of the aurora- and Ipl1-like midbody-associated protein mitotic kinase in human cancer cells. Cancer Res 58:4811–4816
van der Waal MS, Hengeveld RC, van der Horst A, Lens SM (2012) Cell division control by the Chromosomal Passenger Complex. Exp Cell Res 318(12):1407–1420
Lampson MA, Cheeseman IM (2011) Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol 21:133–140
Palazzo RE, Vogel JM, Schnackenberg BJ, Hull DR, Wu X (2000) Centrosome maturation. Curr Top Dev Biol 49:449–470
Mahen R, Venkitaraman AR (2012) Pattern formation in centrosome assembly. Curr Opin Cell Biol 24:14–23
Joukov V, De Nicolo A, Rodriguez A, Walter JC, Livingston DM (2010) Centrosomal protein of 192 kDa (Cep192) promotes centrosome-driven spindle assembly by engaging in organelle-specific Aurora A activation. Proc Natl Acad Sci U S A 107:21022–21027
Hutterer A, Berdnik D, Wirtz-Peitz F, Zigman M, Schleiffer A, Knoblich JA (2006) Mitotic activation of the kinase Aurora-A requires its binding partner Bora. Dev Cell 11:147–157
Pugacheva EN, Golemis EA (2005) The focal adhesion scaffolding protein HEF1 regulates activation of the Aurora-A and Nek2 kinases at the centrosome. Nat Cell Biol 7:937–946
Hannak E, Kirkham M, Hyman AA, Oegema K (2001) Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J Cell Biol 155:1109–1116
Toji S, Yabuta N, Hosomi T, Nishihara S, Kobayashi T, Suzuki S, Tamai K, Nojima H (2004) The centrosomal protein Lats2 is a phosphorylation target of Aurora-A kinase. Genes Cells 9:383–397
Abe Y, Ohsugi M, Haraguchi K, Fujimoto J, Yamamoto T (2006) LATS2-Ajuba complex regulates gamma-tubulin recruitment to centrosomes and spindle organization during mitosis. FEBS Lett 580:782–788
Conte N, Delaval B, Ginestier C, Ferrand A, Isnardon D, Larroque C, Prigent C, Seraphin B, Jacquemier J, Birnbaum D (2003) TACC1-chTOG-Aurora A protein complex in breast cancer. Oncogene 22:8102–8116
Mori D, Yano Y, Toyo-oka K, Yoshida N, Yamada M, Muramatsu M, Zhang D, Saya H, Toyoshima YY, Kinoshita K, Wynshaw-Boris A, Hirotsune S (2007) NDEL1 phosphorylation by Aurora-A kinase is essential for centrosomal maturation, separation, and TACC3 recruitment. Mol Cell Biol 27:352–367
Berdnik D, Knoblich JA (2002) Drosophila Aurora-A is required for centrosome maturation and actin-dependent asymmetric protein localization during mitosis. Curr Biol 12:640–647
De Souza CP, Ellem KA, Gabrielli BG (2000) Centrosomal and cytoplasmic Cdc2/cyclin B1 activation precedes nuclear mitotic events. Exp Cell Res 257:11–21
Jackman M, Lindon C, Nigg EA, Pines J (2003) Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol 5:143–148
Seki A, Coppinger JA, Jang CY, Yates JR, Fang G (2008) Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science 320:1655–1658
Macurek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, Clouin C, Taylor SS, Yaffe MB, Medema RH (2008) Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature 455:119–123
Chan EH, Santamaria A, Sillje HH, Nigg EA (2008) Plk1 regulates mitotic Aurora A function through betaTrCP-dependent degradation of hBora. Chromosoma 117:457–469
Dutertre S, Cazales M, Quaranta M, Froment C, Trabut V, Dozier C, Mirey G, Bouche JP, Theis-Febvre N, Schmitt E, Monsarrat B, Prigent C, Ducommun B (2004) Phosphorylation of CDC25B by Aurora-A at the centrosome contributes to the G2-M transition. J Cell Sci 117:2523–2531
van Vugt MA, Bras A, Medema RH (2004) Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol Cell 15:799–811
Elia AE, Cantley LC, Yaffe MB (2003) Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science 299:1228–1231
Mendez R, Murthy KG, Ryan K, Manley JL, Richter JD (2000) Phosphorylation of CPEB by Eg2 mediates the recruitment of CPSF into an active cytoplasmic polyadenylation complex. Mol Cell 6:1253–1259
Groisman I, Jung MY, Sarkissian M, Cao Q, Richter JD (2002) Translational control of the embryonic cell cycle. Cell 109:473–483
Cao Q, Richter JD (2002) Dissolution of the maskin-eIF4E complex by cytoplasmic polyadenylation and poly(A)-binding protein controls cyclin B1 mRNA translation and oocyte maturation. EMBO J 21:3852–3862
Richter JD (2007) CPEB: a life in translation. Trends Biochem Sci 32:279–285
Groisman I, Huang YS, Mendez R, Cao Q, Theurkauf W, Richter JD (2000) CPEB, maskin, and cyclin B1 mRNA at the mitotic apparatus: implications for local translational control of cell division. Cell 103:435–447
Ouchi M, Fujiuchi N, Sasai K, Katayama H, Minamishima YA, Ongusaha PP, Deng C, Sen S, Lee SW, Ouchi T (2004) BRCA1 phosphorylation by Aurora-A in the regulation of G2 to M transition. J Biol Chem 279:19643–19648
Brodie KM, Henderson BR (2012) Characterization of BRCA1 protein targeting, dynamics, and function at the centrosome: a role for the nuclear export signal, CRM1, and Aurora A kinase. J Biol Chem 287:7701–7716
Kashatus DF, Lim KH, Brady DC, Pershing NL, Cox AD, Counter CM (2011) RALA and RALBP1 regulate mitochondrial fission at mitosis. Nat Cell Biol 13:1108–1115
Liu Q, Ruderman JV (2006) Aurora A, mitotic entry, and spindle bipolarity. Proc Natl Acad Sci U S A 103:5811–5816
Marumoto T, Honda S, Hara T, Nitta M, Hirota T, Kohmura E, Saya H (2003) Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J Biol Chem 278:51786–51795
Katayama H, Sasai K, Kloc M, Brinkley BR, Sen S (2008) Aurora kinase-A regulates kinetochore/chromatin associated microtubule assembly in human cells. Cell Cycle 7:2691–2704
Bird AW, Hyman AA (2008) Building a spindle of the correct length in human cells requires the interaction between TPX2 and Aurora A. J Cell Biol 182:289–300
Barr AR, Gergely F (2007) Aurora-A: the maker and breaker of spindle poles. J Cell Sci 120:2987–2996
Kinoshita K, Noetzel TL, Pelletier L, Mechtler K, Drechsel DN, Schwager A, Lee M, Raff JW, Hyman AA (2005) Aurora A phosphorylation of TACC3/maskin is required for centrosome-dependent microtubule assembly in mitosis. J Cell Biol 170:1047–1055
LeRoy PJ, Hunter JJ, Hoar KM, Burke KE, Shinde V, Ruan J, Bowman D, Galvin K, Ecsedy JA (2007) Localization of human TACC3 to mitotic spindles is mediated by phosphorylation on Ser558 by Aurora A: a novel pharmacodynamic method for measuring Aurora A activity. Cancer Res 67:5362–5370
Ozlu N, Srayko M, Kinoshita K, Habermann B, O’Toole ET, Muller-Reichert T, Schmalz N, Desai A, Hyman AA (2005) An essential function of the C. elegans ortholog of TPX2 is to localize activated aurora A kinase to mitotic spindles. Dev Cell 9:237–248
Barros TP, Kinoshita K, Hyman AA, Raff JW (2005) Aurora A activates D-TACC-Msps complexes exclusively at centrosomes to stabilize centrosomal microtubules. J Cell Biol 170:1039–1046
Zhang X, Ems-McClung SC, Walczak CE (2008) Aurora A phosphorylates MCAK to control ran-dependent spindle bipolarity. Mol Biol Cell 19:2752–2765
Sankaran S, Crone DE, Palazzo RE, Parvin JD (2007) Aurora-A kinase regulates breast cancer associated gene 1 inhibition of centrosome-dependent microtubule nucleation. Cancer Res 67:11186–11194
Chakrabarti R, Jones JL, Oelschlager DK, Tapia T, Tousson A, Grizzle WE (2007) Phosphorylated LIM kinases colocalize with gamma-tubulin in centrosomes during early stages of mitosis. Cell Cycle 6:2944–2952
Ritchey L, Ottman R, Roumanos M, Chakrabarti R (2012) A functional cooperativity between Aurora A kinase and LIM kinase1: implication in the mitotic process. Cell Cycle 11:296–309
Giet R, Uzbekov R, Cubizolles F, Le Guellec K, Prigent C (1999) The Xenopus laevis aurora-related protein kinase pEg2 associates with and phosphorylates the kinesin-related protein XlEg5. J Biol Chem 274:15005–15013
Toso A, Winter JR, Garrod AJ, Amaro AC, Meraldi P, McAinsh AD (2009) Kinetochore-generated pushing forces separate centrosomes during bipolar spindle assembly. J Cell Biol 184:365–372
O’Connell CB, Khodjakov AL (2007) Cooperative mechanisms of mitotic spindle formation. J Cell Sci 120:1717–1722
Tsai MY, Zheng Y (2005) Aurora A kinase-coated beads function as microtubule-organizing centers and enhance RanGTP-induced spindle assembly. Curr Biol 15:2156–2163
Wong J, Lerrigo R, Jang CY, Fang G (2008) Aurora A regulates the activity of HURP by controlling the accessibility of its microtubule-binding domain. Mol Biol Cell 19:2083–2091
Koffa MD, Casanova CM, Santarella R, Kocher T, Wilm M, Mattaj IW (2006) HURP is part of a Ran-dependent complex involved in spindle formation. Curr Biol 16:743–754
Gruss OJ, Vernos I (2004) The mechanism of spindle assembly: functions of Ran and its target TPX2. J Cell Biol 166:949–955
Kim Y, Holland AJ, Lan W, Cleveland DW (2010) Aurora kinases and protein phosphatase 1 mediate chromosome congression through regulation of CENP-E. Cell 142:444–455
Kunitoku N, Sasayama T, Marumoto T, Zhang D, Honda S, Kobayashi O, Hatakeyama K, Ushio Y, Saya H, Hirota T (2003) CENP-A phosphorylation by Aurora-A in prophase is required for enrichment of Aurora-B at inner centromeres and for kinetochore function. Dev Cell 5:853–864
Hegarat N, Smith E, Nayak G, Takeda S, Eyers PA, Hochegger H (2011) Aurora A and Aurora B jointly coordinate chromosome segregation and anaphase microtubule dynamics. J Cell Biol 195:1103–1113
Rong R, Jiang LY, Sheikh MS, Huang Y (2007) Mitotic kinase Aurora-A phosphorylates RASSF1A and modulates RASSF1A-mediated microtubule interaction and M-phase cell cycle regulation. Oncogene 26:7700–7708
Song SJ, Song MS, Kim SJ, Kim SY, Kwon SH, Kim JG, Calvisi DF, Kang D, Lim DS (2009) Aurora A regulates prometaphase progression by inhibiting the ability of RASSF1A to suppress APC-Cdc20 activity. Cancer Res 69:2314–2323
Floyd S, Pines J, Lindon C (2008) APC/C Cdh1 targets aurora kinase to control reorganization of the mitotic spindle at anaphase. Curr Biol 18:1649–1658
Perez de Castro I, Aguirre-Portoles C, Martin B, Fernandez-Miranda G, Klotzbucher A, Kubbutat MHG, Megias D, Arlot-Bonnemains Y, Malumbres M (2011) A SUMOylation motif in Aurora-A: implications fo spindle dynamics and oncogenesis. Front Oncol 1:50. doi:10.3389/fonc.2011.00050
Ma N, Matsunaga S, Morimoto A, Sakashita G, Urano T, Uchiyama S, Fukui K (2011) The nuclear scaffold protein SAF-A is required for kinetochore-microtubule attachment and contributes to the targeting of Aurora-A to mitotic spindles. J Cell Sci 124:394–404
Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM (2007) GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell 12:699–712
Littlepage LE, Wu H, Andresson T, Deanehan JK, Amundadottir LT, Ruderman JV (2002) Identification of phosphorylated residues that affect the activity of the mitotic kinase Aurora-A. Proc Natl Acad Sci U S A 99:15440–15445
Zhao ZS, Lim JP, Ng YW, Lim L, Manser E (2005) The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell 20:237–249
Wittmann T, Wilm M, Karsenti E, Vernos I (2000) TPX2, a novel xenopus MAP involved in spindle pole organization. J Cell Biol 149:1405–1418
Eyers PA, Maller JL (2004) Regulation of Xenopus Aurora A activation by TPX2. J Biol Chem 279:9008–9015
Giubettini M, Asteriti IA, Scrofani J, De Luca M, Lindon C, Lavia P, Guarguaglini G (2011) Control of Aurora-A stability through interaction with TPX2. J Cell Sci 124:113–122
Eyers PA, Erikson E, Chen LG, Maller JL (2003) A novel mechanism for activation of the protein kinase Aurora A. Curr Biol 13:691–697
Kufer TA, Sillje HH, Korner R, Gruss OJ, Meraldi P, Nigg EA (2002) Human TPX2 is required for targeting Aurora-A kinase to the spindle. J Cell Biol 158:617–623
Tsai MY, Wiese C, Cao K, Martin O, Donovan P, Ruderman J, Prigent C, Zheng Y (2003) A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat Cell Biol 5:242–248
Dodson CA, Bayliss R (2012) Activation of Aurora-A kinase by protein partner binding and phosphorylation are independent and synergistic. J Biol Chem 287:1150–1157
Xu X, Wang X, Xiao Z, Li Y, Wang Y (2011) Two TPX2-dependent switches control the activity of Aurora A. PLoS One 6:e16757
Scutt PJ, Chu ML, Sloane DA, Cherry M, Bignell CR, Williams DH, Eyers PA (2009) Discovery and exploitation of inhibitor-resistant aurora and polo kinase mutants for the analysis of mitotic networks. J Biol Chem 284:15880–15893
Manfredi MG, Ecsedy JA, Meetze KA, Balani SK, Burenkova O, Chen W, Galvin KM, Hoar KM, Huck JJ, LeRoy PJ, Ray ET, Sells TB, Stringer B, Stroud SG, Vos TJ, Weatherhead GS, Wysong DR, Zhang M, Bolen JB, Claiborne CF (2007) Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc Natl Acad Sci U S A 104:4106–4111
Eckerdt F, Pascreau G, Phistry M, Lewellyn AL, DePaoli-Roach AA, Maller JL (2009) Phosphorylation of TPX2 by Plx1 enhances activation of Aurora A. Cell Cycle 8:2413–2419
Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M, Hatakeyama K, Saya H (2003) Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell 114:585–598
Sabino D, Brown NH, Basto R (2011) Drosophila Ajuba is not an Aurora-A activator but is required to maintain Aurora-A at the centrosome. J Cell Sci 124:1156–1166
Law SF, Estojak J, Wang B, Mysliwiec T, Kruh G, Golemis EA (1996) Human enhancer of filamentation 1, a novel p130cas-like docking protein, associates with focal adhesion kinase and induces pseudohyphal growth in Saccharomyces cerevisiae. Mol Cell Biol 16:3327–3337
Alexander J, Lim D, Joughin BA, Hegemann B, Hutchins JR, Ehrenberger T, Ivins F, Sessa F, Hudecz O, Nigg EA, Fry AM, Musacchio A, Stukenberg PT, Mechtler K, Peters JM, Smerdon SJ, Yaffe MB (2011) Spatial exclusivity combined with positive and negative selection of phosphorylation motifs is the basis for context-dependent mitotic signaling. Sci Signal 4:ra42
Tikhmyanova N, Little JL, Golemis EA (2010) CAS proteins in normal and pathological cell growth control. Cell Mol Life Sci 67:1025–1048
Astier A, Manie SN, Law SF, Canty T, Haghayghi N, Druker BJ, Salgia R, Golemis EA, Freedman AS (1997) Association of the Cas-like molecule HEF1 with CrkL following integrin and antigen receptor signaling in human B-cells: potential relevance to neoplastic lymphohematopoietic cells. Leuk Lymphoma 28:65–72
Fashena SJ, Einarson MB, O’Neill GM, Patriotis C, Golemis EA (2002) Dissection of HEF1-dependent functions in motility and transcriptional regulation. J Cell Sci 115:99–111
Natarajan M, Stewart JE, Golemis EA, Pugacheva EN, Alexandropoulos K, Cox BD, Wang W, Grammer JR, Gladson CL (2006) HEF1 is a necessary and specific downstream effector of FAK that promotes the migration of glioblastoma cells. Oncogene 25:1721–1732
Kettenbach AN, Schweppe DK, Faherty BK, Pechenick D, Pletnev AA, Gerber SA (2011) Quantitative phosphoproteomics identifies substrates and functional modules of Aurora and Polo-like kinase activities in mitotic cells. Sci Signal 4:rs5
Pugacheva EN, Golemis EA (2006) HEF1-aurora A interactions: points of dialog between the cell cycle and cell attachment signaling networks. Cell Cycle 5:384–391
Dadke D, Jarnik M, Pugacheva EN, Singh MK, Golemis EA (2006) Deregulation of HEF1 impairs M-phase progression by disrupting the RhoA activation cycle. Mol Biol Cell 17:1204–1217
Sankaran S, Starita LM, Groen AC, Ko MJ, Parvin JD (2005) Centrosomal microtubule nucleation activity is inhibited by BRCA1-dependent ubiquitination. Mol Cell Biol 25:8656–8668
Seki A, Coppinger JA, Du H, Jang CY, Yates JR 3rd, Fang G (2008) Plk1- and beta-TrCP-dependent degradation of Bora controls mitotic progression. J Cell Biol 181:65–78
Molli PR, Li DQ, Bagheri-Yarmand R, Pakala SB, Katayama H, Sen S, Iyer J, Chernoff J, Tsai MY, Nair SS, Kumar R (2010) Arpc1b, a centrosomal protein, is both an activator and substrate of Aurora A. J Cell Biol 190:101–114
Francisco L, Wang W, Chan CS (1994) Type 1 protein phosphatase acts in opposition to IpL1 protein kinase in regulating yeast chromosome segregation. Mol Cell Biol 14:4731–4740
Satinover DL, Leach CA, Stukenberg PT, Brautigan DL (2004) Activation of Aurora-A kinase by protein phosphatase inhibitor-2, a bifunctional signaling protein. Proc Natl Acad Sci U S A 101:8625–8630
Katayama H, Zhou H, Li Q, Tatsuka M, Sen S (2001) Interaction and feedback regulation between STK15/BTAK/Aurora-A kinase and protein phosphatase 1 through mitotic cell division cycle. J Biol Chem 276:46219–46224
Zeng K, Bastos RN, Barr FA, Gruneberg U (2010) Protein phosphatase 6 regulates mitotic spindle formation by controlling the T-loop phosphorylation state of Aurora A bound to its activator TPX2. J Cell Biol 191:1315–1332
Reboutier D, Troadec MB, Cremet JY, Fukasawa K, Prigent C (2012) Nucleophosmin/B23 activates Aurora A at the centrosome through phosphorylation of serine 89. J Cell Biol 197:19–26
Plotnikova OV, Pugacheva EN, Dunbrack RL, Golemis EA (2010) Rapid calcium-dependent activation of Aurora-A kinase. Nat Commun 1:64
Sarkissian M, Mendez R, Richter JD (2004) Progesterone and insulin stimulation of CPEB-dependent polyadenylation is regulated by Aurora A and glycogen synthase kinase-3. Gene Dev 18:48–61
Huang YH, Wu CC, Chou CK, Huang CY (2011) A translational regulator, PUM2, promotes both protein stability and kinase activity of Aurora-A. PLoS One 6:e19718
Liu L, Guo C, Dammann R, Tommasi S, Pfeifer GP (2008) RASSF1A interacts with and activates the mitotic kinase Aurora-A. Oncogene 27:6175–6186
Persico A, Cervigni RI, Barretta ML, Corda D, Colanzi A (2010) Golgi partitioning controls mitotic entry through Aurora-A kinase. Mol Biol Cell 21:3708–3721
Fukuda T, Mishina Y, Walker MP, DiAugustine RP (2005) Conditional transgenic system for mouse aurora a kinase: degradation by the ubiquitin proteasome pathway controls the level of the transgenic protein. Mol Cell Biol 25:5270–5281
Honda K, Mihara H, Kato Y, Yamaguchi A, Tanaka H, Yasuda H, Furukawa K, Urano T (2000) Degradation of human Aurora2 protein kinase by the anaphase-promoting complex-ubiquitin-proteasome pathway. Oncogene 19:2812–2819
Walter AO, Seghezzi W, Korver W, Sheung J, Lees E (2000) The mitotic serine/threonine kinase Aurora2/AIK is regulated by phosphorylation and degradation. Oncogene 19:4906–4916
Barford D (2011) Structural insights into anaphase-promoting complex function and mechanism. Philos Trans R Soc Lond B Biol Sci 366:3605–3624
Castro A, Arlot-Bonnemains Y, Vigneron S, Labbe JC, Prigent C, Lorca T (2002) APC/Fizzy-related targets Aurora-A kinase for proteolysis. EMBO Rep 3:457–462
Yu X, Minter-Dykhouse K, Malureanu L, Zhao WM, Zhang D, Merkle CJ, Ward IM, Saya H, Fang G, van Deursen J, Chen J (2005) Chfr is required for tumor suppression and Aurora A regulation. Nat Genet 37:401–406
Littlepage LE, Ruderman JV (2002) Identification of a new APC/C recognition domain, the A box, which is required for the Cdh1-dependent destruction of the kinase Aurora-A during mitotic exit. Gene Dev 16:2274–2285
Horn V, Thelu J, Garcia A, Albiges-Rizo C, Block MR, Viallet J (2007) Functional interaction of Aurora-A and PP2A during mitosis. Mol Biol Cell 18:1233–1241
Pfleger CM, Kirschner MW (2000) The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Gene Dev 14:655–665
Shi Y, Solomon LR, Pereda-Lopez A, Giranda VL, Luo Y, Johnson EF, Shoemaker AR, Leverson J, Liu X (2011) Ubiquitin-specific cysteine protease 2a (USP2a) regulates the stability of Aurora-A. J Biol Chem 286:38960–38968
Kiat LS, Hui KM, Gopalan G (2002) Aurora-A kinase interacting protein (AIP), a novel negative regulator of human Aurora-A kinase. J Biol Chem 277:45558–45565
Lim SK, Gopalan G (2007) Antizyme1 mediates AURKAIP1-dependent degradation of Aurora-A. Oncogene 26:6593–6603
Lim SK, Gopalan G (2007) Aurora-A kinase interacting protein 1 (AURKAIP1) promotes Aurora-A degradation through an alternative ubiquitin-independent pathway. Biochem J 403:119–127
Fumoto K, Lee PC, Saya H, Kikuchi A (2008) AIP regulates stability of Aurora-A at early mitotic phase coordinately with GSK-3beta. Oncogene 27:4478–4487
Kwon YW, Kim IJ, Wu D, Lu J, Stock WA Jr, Liu Y, Huang Y, Kang HC, Delrosario R, Jen KY, Perez-Losada J, Wei G, Balmain A, Mao JH (2012) Pten regulates Aurora-A and cooperates with Fbxw7 in modulating radiation-induced tumor development. Mol Cancer Res 10(6):834–844
Lorenzo C, Liao Q, Hardwicke MA, Ducommun B (2009) Pharmacological inhibition of aurora-A but not aurora-B impairs interphase microtubule dynamics. Cell Cycle 8:1733–1737
Yamada M, Hirotsune S, Wynshaw-Boris A (2010) The essential role of LIS1, NDEL1 and Aurora-A in polarity formation and microtubule organization during neurogenesis. Cell Adh Migr 4:180–184
Rannou Y, Troadec MB, Petretti C, Hans F, Dutertre S, Dimitrov S, Prigent C (2008) Localization of aurora A and aurora B kinases during interphase: role of the N-terminal domain. Cell Cycle 7:3012–3020
Kemp CA, Kopish KR, Zipperlen P, Ahringer J, O’Connell KF (2004) Centrosome maturation and duplication in C. elegans require the coiled-coil protein SPD-2. Dev Cell 6:511–523
Mori D, Yamada M, Mimori-Kiyosue Y, Shirai Y, Suzuki A, Ohno S, Saya H, Wynshaw-Boris A, Hirotsune S (2009) An essential role of the aPKC-Aurora A-NDEL1 pathway in neurite elongation by modulation of microtubule dynamics. Nat Cell Biol 11:1057–1068
Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA (2007) HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129:1351–1363
Plotnikova OV, Pugacheva EN, Golemis EA (2011) Aurora A kinase activity influences calcium signaling in kidney cells. J Cell Biol 193:1021–1032
Toya M, Terasawa M, Nagata K, Iida Y, Sugimoto A (2011) A kinase-independent role for Aurora A in the assembly of mitotic spindle microtubules in Caenorhabditis elegans embryos. Nat Cell Biol 13:708–714
Ogawa H, Ohta N, Moon W, Matsuzaki F (2009) Protein phosphatase 2A negatively regulates aPKC signaling by modulating phosphorylation of Par-6 in Drosophila neuroblast asymmetric divisions. J Cell Sci 122:3242–3249
Zhao B, Smallwood A, Yang J, Koretke K, Nurse K, Calamari A, Kirkpatrick RB, Lai Z (2008) Modulation of kinase-inhibitor interactions by auxiliary protein binding: crystallography studies on Aurora A interactions with VX-680 and with TPX2. Protein Sci 17:1791–1797
Pan J, Snell WJ (2000) Regulated targeting of a protein kinase into an intact flagellum. An aurora/Ipl1p-like protein kinase translocates from the cell body into the flagella during gamete activation in chlamydomonas. J Biol Chem 275:24106–24114
Pan J, Wang Q, Snell WJ (2004) An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev Cell 6:445–451
Plotnikova OV, Golemis EA, Pugacheva EN (2008) Cell cycle-dependent ciliogenesis and cancer. Cancer Res 68:2058–2061
Brush MH, Guardiola A, Connor JH, Yao TP, Shenolikar S (2004) Deactylase inhibitors disrupt cellular complexes containing protein phosphatases and deacetylases. J Biol Chem 279:7685–7691
Kinzel D, Boldt K, Davis EE, Burtscher I, Trumbach D, Diplas B, Attie-Bitach T, Wurst W, Katsanis N, Ueffing M, Lickert H (2010) Pitchfork regulates primary cilia disassembly and left-right asymmetry. Dev Cell 19:66–77
Huang K, Diener DR, Mitchell A, Pazour GJ, Witman GB, Rosenbaum JL (2007) Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J Cell Biol 179:501–514
Wheeler GL, Joint I, Brownlee C (2008) Rapid spatiotemporal patterning of cytosolic Ca2+ underlies flagellar excision in Chlamydomonas reinhardtii. Plant J 53:401–413
Sun L, Hodeify R, Haun S, Charlesworth A, MacNicol AM, Ponnappan S, Ponnappan U, Prigent C, Machaca K (2008) Ca2+ homeostasis regulates Xenopus oocyte maturation. Biol Reprod 78:726–735
Plotnikova OV, Nikonova AS, Loskutov YV, Kozyulina PY, Pugacheva EN, Golemis EA (2012) Calmodulin activation of Aurora-A (AURKA) is required during ciliary disassembly and in mitosis. Mol Biol Cell. doi:10.1091/mbc.E11-12-1056
Harris PC, Torres VE (2009) Polycystic kidney disease. Annu Rev Med 60:321–337
Nowakowski J, Cronin CN, McRee DE, Knuth MW, Nelson CG, Pavletich NP, Rogers J, Sang BC, Scheibe DN, Swanson RV, Thompson DA (2002) Structures of the cancer-related Aurora-A, FAK, and EphA2 protein kinases from nanovolume crystallography. Structure 10:1659–1667
Bayliss R, Sardon T, Vernos I, Conti E (2003) Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol Cell 12:851–862
Zhao B, Smallwood A, Yang J, Koretke K, Nurse K, Calamari A, Kirkpatrick RB, Lai Z (2008) Modulation of kinase-inhibitor interactions by auxiliary protein binding: crystallography studies on Aurora A interactions with VX-680 and with TPX2. Protein Sci 17:1791–1797
Clark MA, Acharya RA, Arico-Muendel CC, Belyanskaya SL, Benjamin DR, Carlson NR, Centrella PA, Chiu CH, Creaser SP, Cuozzo JW, Davie CP, Ding Y, Franklin GJ, Franzen KD, Gefter ML, Hale SP, Hansen NJ, Israel DI, Jiang J, Kavarana MJ, Kelley MS, Kollmann CS, Li F, Lind K, Mataruse S, Medeiros PF, Messer JA, Myers P, O’Keefe H, Oliff MC, Rise CE, Satz AL, Skinner SR, Svendsen JL, Tang L, van Vloten K, Wagner RW, Yao G, Zhao B, Morgan BA (2009) Design, synthesis and selection of DNA-encoded small-molecule libraries. Nat Chem Biol 5:647–654
Dodson CA, Kosmopoulou M, Richards MW, Atrash B, Bavetsias V, Blagg J, Bayliss R (2010) Crystal structure of an Aurora-A mutant that mimics Aurora-B bound to MLN8054: insights into selectivity and drug design. Biochem J 427:19–28
Kornev AP, Taylor SS (2010) Defining the conserved internal architecture of a protein kinase. Biochim Biophys Acta 1804:440–444
Heron NM, Anderson M, Blowers DP, Breed J, Eden JM, Green S, Hill GB, Johnson T, Jung FH, McMiken HH, Mortlock AA, Pannifer AD, Pauptit RA, Pink J, Roberts NJ, Rowsell S (2006) SAR and inhibitor complex structure determination of a novel class of potent and specific Aurora kinase inhibitors. Bioorg Med Chem Lett 16:1320–1323
Bibby RA, Tang C, Faisal A, Drosopoulos K, Lubbe S, Houlston R, Bayliss R, Linardopoulos S (2009) A cancer-associated aurora A mutant is mislocalized and misregulated due to loss of interaction with TPX2. J Biol Chem 284:33177–33184
Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, Chan CS, Novotny M, Slamon DJ, Plowman GD (1998) A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J 17:3052–3065
Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, Brinkley BR, Sen S (1998) Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet 20:189–193
Jiang S, Katayama H, Wang J, Li SA, Hong Y, Radvanyi L, Li JJ, Sen S (2010) Estrogen-induced aurora kinase-A (AURKA) gene expression is activated by GATA-3 in estrogen receptor-positive breast cancer cells. Horm Cancer 1:11–20
Lee HH, Zhu Y, Govindasamy KM, Gopalan G (2008) Downregulation of Aurora-A overrides estrogen-mediated growth and chemoresistance in breast cancer cells. Endocr Relat Cancer 15:765–775
Xu J, Li H, Wang B, Xu Y, Yang J, Zhang X, Harten SK, Shukla D, Maxwell PH, Pei D, Esteban MA (2010) VHL inactivation induces HEF1 and Aurora kinase A. J Am Soc Nephrol 21:2041–2046
Karthigeyan D, Prasad SB, Shandilya J, Agrawal S, Kundu TK (2011) Biology of Aurora A kinase: implications in cancer manifestation and therapy. Med Res Rev 31(5):757–793
Yin N, Shi J, Wang D, Tong T, Wang M, Fan F, Zhan Q (2012) IQGAP1 interacts with Aurora-A and enhances its stability and its role in cancer. Biochem Biophys Res Commun 421:64–69
Aguirre-Portoles C, Bird AW, Hyman A, Canamero M, Perez de Castro I, Malumbres M (2012) Tpx2 controls spindle integrity, genome stability, and tumor development. Cancer Res 72:1518–1528
Sanbhnani S, Yeong FM (2012) CHFR: a key checkpoint component implicated in a wide range of cancers. Cell Mol Life Sci 69:1669–1687
Asteriti IA, Rensen WM, Lindon C, Lavia P, Guarguaglini G (2010) The Aurora-A/TPX2 complex: a novel oncogenic holoenzyme? Biochim Biophys Acta 1806:230–239
Dar AA, Goff LW, Majid S, Berlin J, El-Rifai W (2010) Aurora kinase inhibitors—rising stars in cancer therapeutics? Mol Cancer Ther 9:268–278
Gautschi O, Heighway J, Mack PC, Purnell PR, Lara PN Jr, Gandara DR (2008) Aurora kinases as anticancer drug targets. Clin Cancer Res 14:1639–1648
Katayama H, Brinkley WR, Sen S (2003) The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev 22:451–464
Marumoto T, Zhang D, Saya H (2005) Aurora-A—a guardian of poles. Nat Rev Cancer 5:42–50
Yan A, Wang L, Xu S, Xu J (2011) Aurora-A kinase inhibitor scaffolds and binding modes. Drug Discov Today 16:260–269
Zhang D, Shimizu T, Araki N, Hirota T, Yoshie M, Ogawa K, Nakagata N, Takeya M, Saya H (2008) Aurora A overexpression induces cellular senescence in mammary gland hyperplastic tumors developed in p53-deficient mice. Oncogene 27:4305–4314
Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF (1996) Abnormal centrosome amplification in the absence of p53. Science 271:1744–1747
Donehower LA, Godley LA, Aldaz CM, Pyle R, Shi YP, Pinkel D, Gray J, Bradley A, Medina D, Varmus HE (1995) Deficiency of p53 accelerates mammary tumorigenesis in Wnt-1 transgenic mice and promotes chromosomal instability. Genes Dev 9:882–895
Katayama H, Sasai K, Kawai H, Yuan ZM, Bondaruk J, Suzuki F, Fujii S, Arlinghaus RB, Czerniak BA, Sen S (2004) Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet 36:55–62
Chen SS, Chang PC, Cheng YW, Tang FM, Lin YS (2002) Suppression of the STK15 oncogenic activity requires a transactivation-independent p53 function. EMBO J 21:4491–4499
Mao JH, Wu D, Perez-Losada J, Jiang T, Li Q, Neve RM, Gray JW, Cai WW, Balmain A (2007) Crosstalk between Aurora-A and p53: frequent deletion or downregulation of Aurora-A in tumors from p53 null mice. Cancer Cell 11:161–173
Mao JH, Perez-Losada J, Wu D, Delrosario R, Tsunematsu R, Nakayama KI, Brown K, Bryson S, Balmain A (2004) Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature 432:775–779
Cai QL, Knight JS, Verma SC, Zald P, Robertson ES (2006) EC5S ubiquitin complex is recruited by KSHV latent antigen LANA for degradation of the VHL and p53 tumor suppressors. PLoS Pathog 2:e116
Cai Q, Xiao B, Si H, Cervini A, Gao J, Lu J, Upadhyay SK, Verma SC, Robertson ES (2012) Kaposi’s sarcoma herpesvirus upregulates Aurora A expression to promote p53 phosphorylation and ubiquitylation. PLoS Pathog 8:e1002566
Katayama H, Wang J, Treekitkarnmongkol W, Kawai H, Sasai K, Zhang H, Wang H, Adams HP, Jiang S, Chakraborty SN, Suzuki F, Arlinghaus RB, Liu J, Mobley JA, Grizzle WE, Sen S (2012) Aurora kinase-A inactivates DNA damage-induced apoptosis and spindle assembly checkpoint response functions of p73. Cancer Cell 21:196–211
Zou L, Sun Y, Wang M, Zhan Q (2011) Aurora-A interacts with AP-2alpha and down regulates its transcription activity. PloS One 6:e23110
Briassouli P, Chan F, Savage K, Reis-Filho JS, Linardopoulos S (2007) Aurora-A regulation of nuclear factor-kappaB signaling by phosphorylation of IkappaBalpha. Cancer Res 67:1689–1695
Chefetz I, Holmberg JC, Alvero AB, Visintin I, Mor G (2011) Inhibition of Aurora-A kinase induces cell cycle arrest in epithelial ovarian cancer stem cells by affecting NFkB pathway. Cell Cycle 10:2206–2214
Yang H, He L, Kruk P, Nicosia SV, Cheng JQ (2006) Aurora-A induces cell survival and chemoresistance by activation of Akt through a p53-dependent manner in ovarian cancer cells. J Int Cancer 119:2304–2312
Lim KH, Brady DC, Kashatus DF, Ancrile BB, Der CJ, Cox AD, Counter CM (2010) Aurora-A phosphorylates, activates, and relocalizes the small GTPase RalA. Mol Cell Biol 30:508–523
Wu JC, Chen TY, Yu CT, Tsai SJ, Hsu JM, Tang MJ, Chou CK, Lin WJ, Yuan CJ, Huang CY (2005) Identification of V23RalA-Ser194 as a critical mediator for Aurora-A-induced cellular motility and transformation by small pool expression screening. J Biol Chem 280:9013–9022
Neben K, Korshunov A, Benner A, Wrobel G, Hahn M, Kokocinski F, Golanov A, Joos S, Lichter P (2004) Microarray-based screening for molecular markers in medulloblastoma revealed STK15 as independent predictor for survival. Cancer Res 64:3103–3111
Dimova I, Raitcheva S, Dimitrov R, Doganov N, Toncheva D (2006) Correlations between c-myc gene copy-number and clinicopathological parameters of ovarian tumours. Eur J Cancer 42:674–679
Lassmann S, Shen Y, Jutting U, Wiehle P, Walch A, Gitsch G, Hasenburg A, Werner M (2007) Predictive value of Aurora-A/STK15 expression for late stage epithelial ovarian cancer patients treated by adjuvant chemotherapy. Clin Cancer Res 13:4083–4091
Otto T, Horn S, Brockmann M, Eilers U, Schuttrumpf L, Popov N, Kenney AM, Schulte JH, Beijersbergen R, Christiansen H, Berwanger B, Eilers M (2009) Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell 15:67–78
Beltran H, Rickman DS, Park K, Chae SS, Sboner A, Macdonald TY, Wang Y, Sheikh KL, Terry S, Tagawa ST, Dhir R, Nelson JB, de la Taille A, Allory Y, Gerstein MB, Perner S, Pienta KJ, Chinnaiyan AM, Collins CC, Gleave ME, Demichelis F, Nanus DM, Rubin MA (2011) Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 1:487–495
Yang H, Ou CC, Feldman RI, Nicosia SV, Kruk PA, Cheng JQ (2004) Aurora-A kinase regulates telomerase activity through c-Myc in human ovarian and breast epithelial cells. Cancer Res 64:463–467
Yang S, He S, Zhou X, Liu M, Zhu H, Wang Y, Zhang W, Yan S, Quan L, Bai J, Xu N (2010) Suppression of Aurora-A oncogenic potential by c-Myc downregulation. Exp Mol Med 42:759–767
Dar AA, Belkhiri A, El-Rifai W (2009) The aurora kinase A regulates GSK-3beta in gastric cancer cells. Oncogene 28:866–875
den Hollander J, Rimpi S, Doherty JR, Rudelius M, Buck A, Hoellein A, Kremer M, Graf N, Scheerer M, Hall MA, Goga A, von Bubnoff N, Duyster J, Peschel C, Cleveland JL, Nilsson JA, Keller U (2010) Aurora kinases A and B are up-regulated by Myc and are essential for maintenance of the malignant state. Blood 116:1498–1505
Sumi K, Tago K, Kasahara T, Funakoshi-Tago M (2011) Aurora kinase A critically contributes to the resistance to anti-cancer drug cisplatin in JAK2 V617F mutant-induced transformed cells. FEBS Lett 585:1884–1890
Torchia EC, Caulin C, Acin S, Terzian T, Kubick BJ, Box NF, Roop DR (2012) Myc, Aurora Kinase A, and mutant p53(R172H) co-operate in a mouse model of metastatic skin carcinoma. Oncogene 31:2680–2690
Maxwell CA, Benitez J, Gomez-Baldo L, Osorio A, Bonifaci N, Fernandez-Ramires R, Costes SV, Guino E, Chen H, Evans GJ, Mohan P, Catala I, Petit A, Aguilar H, Villanueva A, Aytes A, Serra-Musach J, Rennert G, Lejbkowicz F, Peterlongo P, Manoukian S, Peissel B, Ripamonti CB, Bonanni B, Viel A, Allavena A, Bernard L, Radice P, Friedman E, Kaufman B, Laitman Y, Dubrovsky M, Milgrom R, Jakubowska A, Cybulski C, Gorski B, Jaworska K, Durda K, Sukiennicki G, Lubinski J, Shugart YY, Domchek SM, Letrero R, Weber BL, Hogervorst FB, Rookus MA, Collee JM, Devilee P, Ligtenberg MJ, Luijt RB, Aalfs CM, Waisfisz Q, Wijnen J, Roozendaal CE, Easton DF, Peock S, Cook M, Oliver C, Frost D, Harrington P, Evans DG, Lalloo F, Eeles R, Izatt L, Chu C, Eccles D, Douglas F, Brewer C, Nevanlinna H, Heikkinen T, Couch FJ, Lindor NM, Wang X, Godwin AK, Caligo MA, Lombardi G, Loman N, Karlsson P, Ehrencrona H, Wachenfeldt A, Barkardottir RB, Hamann U, Rashid MU, Lasa A, Caldes T, Andres R, Schmitt M, Assmann V, Stevens K, Offit K, Curado J, Tilgner H, Guigo R, Aiza G, Brunet J, Castellsague J, Martrat G, Urruticoechea A, Blanco I, Tihomirova L, Goldgar DE, Buys S, John EM, Miron A, Southey M, Daly MB, Schmutzler RK, Wappenschmidt B, Meindl A, Arnold N, Deissler H, Varon-Mateeva R, Sutter C, Niederacher D, Imyamitov E, Sinilnikova OM, Stoppa-Lyonne D, Mazoyer S, Verny-Pierre C, Castera L, de Pauw A, Bignon YJ, Uhrhammer N, Peyrat JP, Vennin P, Fert Ferrer S, Collonge-Rame MA, Mortemousque I, Spurdle AB, Beesley J, Chen X, Healey S, Barcellos-Hoff MH, Vidal M, Gruber SB, Lazaro C, Capella G, McGuffog L, Nathanson KL, Antoniou AC, Chenevix-Trench G, Fleisch MC, Moreno V, Pujana MA (2011) Interplay between BRCA1 and RHAMM regulates epithelial apicobasal polarization and may influence risk of breast cancer. PLoS Biol 9:e1001199
Ratushny V, Pathak HB, Beeharry N, Tikhmyanova N, Xiao F, Li T, Litwin S, Connolly DC, Yen TJ, Weiner LM, Godwin AK, Golemis EA (2011) Dual inhibition of SRC and Aurora kinases induces postmitotic attachment defects and cell death. Oncogene 31(10):1217–1227. doi:10.1038/onc.2011.314
Arai R, Tsuda M, Watanabe T, Ose T, Obuse C, Maenaka K, Minami A, Ohba Y (2012) Simultaneous inhibition of Src and Aurora kinases by SU6656 induces therapeutic synergy in human synovial sarcoma growth, invasion and angiogenesis in vivo. Eur J Cancer. doi:10.1016/j.ejca.2011.12.028
Gritsko TM, Coppola D, Paciga JE, Yang L, Sun M, Shelley SA, Fiorica JV, Nicosia SV, Cheng JQ (2003) Activation and overexpression of centrosome kinase BTAK/Aurora-A in human ovarian cancer. Clin Cancer Res 9:1420–1426
Hu W, Kavanagh JJ, Deaver M, Johnston DA, Freedman RS, Verschraegen CF, Sen S (2005) Frequent overexpression of STK15/Aurora-A/BTAK and chromosomal instability in tumorigenic cell cultures derived from human ovarian cancer. Oncol Res 15:49–57
Tanner MM, Grenman S, Koul A, Johannsson O, Meltzer P, Pejovic T, Borg A, Isola JJ (2000) Frequent amplification of chromosomal region 20q12–q13 in ovarian cancer. Clin Cancer Res 6:1833–1839
Kulkarni AA, Loddo M, Leo E, Rashid M, Eward KL, Fanshawe TR, Butcher J, Frost A, Ledermann JA, Williams GH, Stoeber K (2007) DNA replication licensing factors and aurora kinases are linked to aneuploidy and clinical outcome in epithelial ovarian carcinoma. Clin Cancer Res 13:6153–6161
Landen CN Jr, Lin YG, Immaneni A, Deavers MT, Merritt WM, Spannuth WA, Bodurka DC, Gershenson DM, Brinkley WR, Sood AK (2007) Overexpression of the centrosomal protein Aurora-A kinase is associated with poor prognosis in epithelial ovarian cancer patients. Clin Cancer Res 13:4098–4104
Das K, Lorena PD, Ng LK, Shen L, Lim D, Siow WY, Narasimhan K, Teh M, Choolani M, Putti TC, Salto-Tellez M (2010) Aurora-A expression, hormone receptor status and clinical outcome in hormone related cancers. Pathology 42:540–546
Lassus H, Staff S, Leminen A, Isola J, Butzow R (2011) Aurora-A overexpression and aneuploidy predict poor outcome in serous ovarian carcinoma. Gynecol Oncol 120:11–17
Mendiola M, Barriuso J, Marino-Enriquez A, Redondo A, Dominguez-Caceres A, Hernandez-Cortes G, Perez-Fernandez E, Sanchez-Navarro I, Vara JA, Suarez A, Espinosa E, Gonzalez-Baron M, Palacios J, Hardisson D (2009) Aurora kinases as prognostic biomarkers in ovarian carcinoma. Hum Pathol 40:631–638
Yang G, Chang B, Yang F, Guo X, Cai KQ, Xiao XS, Wang H, Sen S, Hung MC, Mills GB, Chang S, Multani AS, Mercado-Uribe I, Liu J (2010) Aurora kinase A promotes ovarian tumorigenesis through dysregulation of the cell cycle and suppression of BRCA2. Clin Cancer Res 16:3171–3181
Yang F, Guo X, Yang G, Rosen DG, Liu J (2011) AURKA and BRCA2 expression highly correlate with prognosis of endometrioid ovarian carcinoma. Mod Pathol 24:836–845
Carvalho B, Postma C, Mongera S, Hopmans E, Diskin S, van de Wiel MA, van Criekinge W, Thas O, Matthai A, Cuesta MA, Terhaar Sive Droste JS, Craanen M, Schrock E, Ylstra B, Meijer GA (2009) Multiple putative oncogenes at the chromosome 20q amplicon contribute to colorectal adenoma to carcinoma progression. Gut 58:79–89
Sillars-Hardebol AH, Carvalho B, Tijssen M, Belien JA, de Wit M, Delis-van Diemen PM, Ponten F, van de Wiel MA, Fijneman RJ, Meijer GA (2011) TPX2 and AURKA promote 20q amplicon-driven colorectal adenoma to carcinoma progression. Gut. doi:10.1136/gutjnl-2011-301153
Baba Y, Nosho K, Shima K, Irahara N, Kure S, Toyoda S, Kirkner GJ, Goel A, Fuchs CS, Ogino S (2009) Aurora-A expression is independently associated with chromosomal instability in colorectal cancer. Neoplasia 11:418–425
Belt EJ, Brosens RP, Delis-van Diemen PM, Bril H, Tijssen M, van Essen DF, Heymans MW, Belien JA, Stockmann HB, Meijer S, Meijer GA (2012) Cell cycle proteins predict recurrence in stage II and III colon cancer. Ann Surg Oncol. doi:10.1245/s10434-012-2216-7
Lam AK, Ong K, Ho YH (2008) Aurora kinase expression in colorectal adenocarcinoma: correlations with clinicopathological features, p16 expression, and telomerase activity. Hum Pathol 39:599–604
Dotan E, Meropol NJ, Zhu F, Zambito F, Bove B, Cai KQ, Godwin AK, Golemis EA, Astsaturov I, Cohen SJ (2012) Relationship of increased aurora kinase A gene copy number, prognosis and response to chemotherapy in patients with metastatic colorectal cancer. Br J Cancer 106:748–755
Ewart-Toland A, Briassouli P, de Koning JP, Mao JH, Yuan J, Chan F, MacCarthy-Morrogh L, Ponder BA, Nagase H, Burn J, Ball S, Almeida M, Linardopoulos S, Balmain A (2003) Identification of Stk6/STK15 as a candidate low-penetrance tumor-susceptibility gene in mouse and human. Nat Genet 34:403–412
Ewart-Toland A, Dai Q, Gao YT, Nagase H, Dunlop MG, Farrington SM, Barnetson RA, Anton-Culver H, Peel D, Ziogas A, Lin D, Miao X, Sun T, Ostrander EA, Stanford JL, Langlois M, Chan JM, Yuan J, Harris CC, Bowman ED, Clayman GL, Lippman SM, Lee JJ, Zheng W, Balmain A (2005) Aurora-A/STK15 T + 91A is a general low penetrance cancer susceptibility gene: a meta-analysis of multiple cancer types. Carcinogenesis 26:1368–1373
Nadler Y, Camp RL, Schwartz C, Rimm DL, Kluger HM, Kluger Y (2008) Expression of Aurora A (but not Aurora B) is predictive of survival in breast cancer. Clin Cancer Res 14:4455–4462
Dai Q, Cai QY, Shu XO, Ewart-Toland A, Wen WQ, Balmain A, Gao YT, Zheng W (2004) Synergistic effects of STK15 gene polymorphisms and endogenous estrogen exposure in the risk of breast cancer. Cancer Epidemiol Biomark Prev 13:2065–2070
Lo YL, Yu JC, Chen ST, Yang HC, Fann CS, Mau YC, Shen CY (2005) Breast cancer risk associated with genotypic polymorphism of the mitosis-regulating gene Aurora-A/STK15/BTAK. J Int Cancer 115:276–283
Cox DG, Hankinson SE, Hunter DJ (2006) Polymorphisms of the AURKA (STK15/Aurora Kinase) gene and breast cancer risk (United States). Cancer Causes Control 17:81–83
Ogawa E, Takenaka K, Katakura H, Adachi M, Otake Y, Toda Y, Kotani H, Manabe T, Wada H, Tanaka F (2008) Perimembrane Aurora-A expression is a significant prognostic factor in correlation with proliferative activity in non-small-cell lung cancer (NSCLC). Ann Surg Oncol 15:547–554
Lo Iacono M, Monica V, Saviozzi S, Ceppi P, Bracco E, Papotti M, Scagliotti GV (2011) Aurora kinase A expression is associated with lung cancer histological-subtypes and with tumor de-differentiation. J Transl Med 9:100
Gu J, Gong Y, Huang M, Lu C, Spitz MR, Wu X (2007) Polymorphisms of STK15 (Aurora-A) gene and lung cancer risk in Caucasians. Carcinogenesis 28:350–355
Yu CT, Hsia JY, Hseih YC, Su LJ, Lee TC, Ku CF, Chen KS, Chen JM, Wei TY, Lee YC, Huang CY, Wu YC, Yang CY, Hsu SL (2011) The novel protein suppressed in lung cancer down-regulated in lung cancer tissues retards cell proliferation and inhibits the oncokinase Aurora-A. J Thorac Oncol 6:988–997
Reiter R, Gais P, Jutting U, Steuer-Vogt MK, Pickhard A, Bink K, Rauser S, Lassmann S, Hofler H, Werner M, Walch A (2006) Aurora kinase A messenger RNA overexpression is correlated with tumor progression and shortened survival in head and neck squamous cell carcinoma. Clin Cancer Res 12:5136–5141
Kollareddy M, Zheleva D, Dzubak P, Brahmkshatriya PS, Lepsik M, Hajduch M (2012) Aurora kinase inhibitors: progress towards the clinic. Investig New Drugs. doi:10.1007/s10637-012-9798-6
Sonet A, Graux C, Maertens J, Hartog C-M, Duyster J, Gotze K, Greiner J, Hutter M-, Gratwohl A, Heim D, Hess D, Chalandon Y, Gianella-Borradori A, Rejeb N, Ottman O (2008) Phase I, dose-escalation study of 2 dosing regimens of AS703569, an inhibitor of aurora and other kinases, administered orally in patients with advanced hematological malignancies. Blood ASH Ann Meet Abstr 112:2963
Chakravarty A, Shinde V, Tabernero J, Cervantes A, Cohen RB, Dees EC, Burris H, Infante JR, Macarulla T, Elez E, Andreu J, Rodriguez-Braun E, Rosello S, von Mehren M, Meropol NJ, Langer CJ, ONeil B, Bowman D, Zhang M, Danaee H, Faron-Yowe L, Gray G, Liu H, Pappas J, Silverman L, Simpson C, Stringer B, Tirrell S, Veiby OP, Venkatakrishnan K, Galvin K, Manfredi M, Ecsedy JA (2011) Phase I assessment of new mechanism-based pharmacodynamic biomarkers for MLN8054, a small-molecule inhibitor of Aurora A kinase. Cancer Res 71:675–685
Dees EC, Infante JR, Cohen RB, O’Neil BH, Jones S, von Mehren M, Danaee H, Lee Y, Ecsedy J, Manfredi M, Galvin K, Stringer B, Liu H, Eton O, Fingert H, Burris H (2011) Phase 1 study of MLN8054, a selective inhibitor of Aurora A kinase in patients with advanced solid tumors. Cancer Chemother Pharmacol 67:945–954
Macarulla T, Cervantes A, Elez E, Rodriguez-Braun E, Baselga J, Rosello S, Sala G, Blasco I, Danaee H, Lee Y, Ecsedy J, Shinde V, Chakravarty A, Bowman D, Liu H, Eton O, Fingert H, Tabernero J (2010) Phase I study of the selective Aurora A kinase inhibitor MLN8054 in patients with advanced solid tumors: safety, pharmacokinetics, and pharmacodynamics. Mol Cancer Ther 9:2844–2852
Kelly KR, Nawrocki ST, Espitia CM, Zhang M, Yang JJ, Padmanabhan S, Ecsedy J, Giles FJ, Carew JS (2012) Targeting aurora a kinase activity with the investigational agent alisertib increases the efficacy of cytarabine through a FOXO-dependent mechanism. Int J Cancer. doi:10.1002/ijc.27579
Goldberg SL, Fenaux P, Craig MD, Gyan E, Lister J, Kassis J, Pigneux A, Schiller GJ, Jung J, Leonard EJ, Fingert H, Westervelt P (2010) Phase 2 study of MLN8237, an investigational Aurora A kinase (AAK) inhibitor in patients with acute myelogenous leukemia (AML) or myelodysplastic syndromes (MDS). ASH Ann Meet Abstr 116:3273
Friedberg J, Mahadevan D, Jung J, Persky DO, Lossos IS, Danaee H, Zhou X, Leonard EJ, Bernstein SH (2011) Phase 2 trial of alisertib (MLN8237), an investigational, potent inhibitor of Aurora A kinase (AAK), in patients (pts) with aggressive B- and T-cell non-hodgkin lymphoma (NHL). ASH Ann Meet Abstr 118:95
Mehra R, Serebriiskii IG, Dunbrack RL Jr, Robinson MK, Burtness B, Golemis EA (2011) Protein-intrinsic and signaling network-based sources of resistance to EGFR- and ErbB family-targeted therapies in head and neck cancer. Drug Resist Updat 14:260–279
O’Connor O, Leonard EJ, Benaim E (2012) Phase III study of investigational MLN8237 (alisertib) versus investigator’s choice in patients (pts) with relapsed/refractory (rel/ref) peripheral T-cell lymphoma (PTCL). J Clin Oncol 30(suppl); abstr TPS8110. http://www.asco.org/ASCOv2/Meetings/Abstract?&vmview=abst_detail_view&conflD=114&abstractID=93843
Heiser LM, Sadanandam A, Kuo WL, Benz SC, Goldstein TC, Ng S, Gibb WJ, Wang NJ, Ziyad S, Tong F, Bayani N, Hu Z, Billig JI, Dueregger A, Lewis S, Jakkula L, Korkola JE, Durinck S, Pepin F, Guan Y, Purdom E, Neuvial P, Bengtsson H, Wood KW, Smith PG, Vassilev LT, Hennessy BT, Greshock J, Bachman KE, Hardwicke MA, Park JW, Marton LJ, Wolf DM, Collisson EA, Neve RM, Mills GB, Speed TP, Feiler HS, Wooster RF, Haussler D, Stuart JM, Gray JW, Spellman PT (2012) Subtype and pathway specific responses to anticancer compounds in breast cancer. Proc Natl Acad Sci U S A 109:2724–2729
Cohen RB, Jones SF, Aggarwal C, von Mehren M, Cheng J, Spigel DR, Greco FA, Mariani M, Rocchetti M, Ceruti R, Comis S, Laffranchi B, Moll J, Burris HA (2009) A phase I dose-escalation study of danusertib (PHA-739358) administered as a 24-hour infusion with and without granulocyte colony-stimulating factor in a 14-day cycle in patients with advanced solid tumors. Clin Cancer Res 15:6694–6701
Loeb LA (2011) Human cancers express mutator phenotypes: origin, consequences and targeting. Nat Rev Cancer 11:450–457
Venkataraman S, Alimova I, Tello T, Harris PS, Knipstein JA, Donson AM, Foreman NK, Liu AK, Vibhakar R (2012) Targeting Aurora Kinase A enhances radiation sensitivity of atypical teratoid rhabdoid tumor cells. J Neurooncol 107:517–526
Guan Z, Wang XR, Zhu XF, Huang XF, Xu J, Wang LH, Wan XB, Long ZJ, Liu JN, Feng GK, Huang W, Zeng YX, Chen FJ, Liu Q (2007) Aurora-A, a negative prognostic marker, increases migration and decreases radiosensitivity in cancer cells. Cancer Res 67:10436–10444
Astsaturov I, Ratushny V, Sukhanova A, Einarson MB, Bagnyukova T, Zhou Y, Devarajan K, Silverman JS, Tikhmyanova N, Skobeleva N, Pecherskaya A, Nasto RE, Sharma C, Jablonski SA, Serebriiskii IG, Weiner LM, Golemis EA (2010) Synthetic lethal screen of an EGFR-centered network to improve targeted therapies. Sci Signal 3:ra67
Shao S, Wang Y, Jin S, Song Y, Wang X, Fan W, Zhao Z, Fu M, Tong T, Dong L, Fan F, Xu N, Zhan Q (2006) Gadd45a interacts with aurora-A and inhibits its kinase activity. J Biol Chem 281:28943–28950
Lukasiewicz KB, Greenwood TM, Negron VC, Bruzek AK, Salisbury JL, Lingle WL (2011) Control of centrin stability by Aurora A. PloS One 6:e21291
Mosquera J, Armisen R, Zhao H, Rojas DA, Maldonado E, Tapia JC, Colombo A, Hayman MJ, Marcelain K (2011) Identification of Ski as a target for Aurora A kinase. Biochem Biophys Res Commun 409:539–543
Liu Q, Kaneko S, Yang L, Feldman RI, Nicosia SV, Chen J, Cheng JQ (2004) Aurora-A abrogation of p53 DNA binding and transactivation activity by phosphorylation of serine 215. J Biol Chem 279:52175–52182
Scrittori L, Hans F, Angelov D, Charra M, Prigent C, Dimitrov S (2001) pEg2 aurora-A kinase, histone H3 phosphorylation, and chromosome assembly in Xenopus egg extract. J Biol Chem 276:30002–30010
Sasayama T, Marumoto T, Kunitoku N, Zhang D, Tamaki N, Kohmura E, Saya H, Hirota T (2005) Over-expression of Aurora-A targets cytoplasmic polyadenylation element binding protein and promotes mRNA polyadenylation of Cdk1 and cyclin B1. Genes Cells 10:627–638
Johnson EO, Chang KH, Ghosh S, Venkatesh C, Giger K, Low PS, Shah K (2012) LIMK2 is a crucial regulator and effector of Aurora-A-kinase-mediated malignancy. J Cell Sci 125:1204–1216
Terada Y, Uetake Y, Kuriyama R (2003) Interaction of Aurora-A and centrosomin at the microtubule-nucleating site in Drosophila and mammalian cells. J Cell Biol 162:757–763
Jang MS, Sul JW, Choi BJ, Lee SJ, Suh JH, Kim NS, Kim WH, Lim DS, Lee CW, Kim E (2008) Negative feedback regulation of Aurora-A via phosphorylation of Fas-associated factor-1. J Biol Chem 283:32344–32351
Johnson EO, Chang KH, de Pablo Y, Ghosh S, Mehta R, Badve S, Shah K (2011) PHLDA1 is a crucial negative regulator and effector of Aurora A kinase in breast cancer. J Cell Sci 124:2711–2722
Szklarczyk D, Franceschini A, Kuhn M, Simonovic M, Roth A, Minguez P, Doerks T, Stark M, Muller J, Bork P, Jensen LJ, von Mering C (2011) The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res 39:D561–D568
Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T (2011) Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics (Oxford, England) 27:431–432
Acknowledgments
The authors were supported by R01s CA63366 and CA113342 (to EAG); by NIH R21 CA-164205 and 1K22CA-160725 (to IA); NIH R01 GM84453 (to RLD); and NIH core grant CA-06927, the Commonwealth of Pennsylvania, and the Pew Charitable Fund (to Fox Chase Cancer Center). The authors apologize for not being able to include discussion of additional relevant Aurora-A-related studies, because of the length limitations of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nikonova, A.S., Astsaturov, I., Serebriiskii, I.G. et al. Aurora A kinase (AURKA) in normal and pathological cell division. Cell. Mol. Life Sci. 70, 661–687 (2013). https://doi.org/10.1007/s00018-012-1073-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1073-7