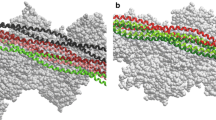
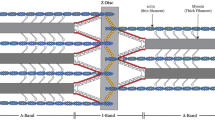
Summary
An accurate value for mass/length of thick myofilaments is required to establish a limit for the maximum number of myosin molecules per crossbridge repeat. The mass/length of the crossbridge regions of desalted thick myofilaments from insect flight muscle (Lethocerus andMusca) and rabbit psoas has been measured with a computer-linked STEM by comparing the electron scattering signal per unit length of unstained thick filaments with that from TMV particles in the same image. Filament preparation was aided by limited digestion of myofibrils to remove Z bands using calcium-activated factor (CAF) from rabbit skeletal muscle; SDS gels showed that this selective protease spared myosin and tended to spare paramyosin but removed C protein.Lethocerus filaments prepared by the CAF procedure were 20–25% heavier per unit length than those prepared by conventional (simple) shearing, and retained a clear and generally uniform 14.5 nm crossbridge repeat by negative staining.
We have expressed mass/length of thick filaments as myosin equivalents (mol. wt 0.470 × 106) per crown (that is, the 14.5 nm insect or 14.3 nm vertebrate repeat along thick filaments). TMV standards, calculated to weigh 0.1304×106 daltons nm−1 and thus equivalent to 4.02 myosins per 14.5 nm, were uniform to ±3%s.d. for 73 particles after normalizing means for each different image field. After subtracting the known paramyosin content from insect measurements (11% for waterbug, 2% for the housefly), but making no C protein correction to rabbit measurements, the following results were obtained:Lethocerus (all) 4.19±0.50 (243 filaments);Lethocerus (CAF prepns) 4.40±0.44 (145 filaments);Musca (all CAF) 4.14±0.37 (57 filaments); rabbit (all CAF) 2.86±0.34 (75 filaments).
These values favour the lowest integral number of myosins per crown among currently competing models of thick filament structure. The rabbit value agrees with several previous estimates, including the STEM measurements of Lamvik, which indicated three rather than four myosins/crown. The insect flight myofilament value of four forces re-evaluation of previous estimates by quantitative gels and quantitative microscopy of whole fibrils which had favoured six myosins/crown.
Similar content being viewed by others
References
ABBOTT, R. H. & CAGE, P. E. (1978) Periodicity in insect flight muscle stretch activation.J. Physiol. 231, 195–208.
AUST, S., HINKEL, P. & BEINBRECH, G. (1980) Evidence for four-stranded myosin filaments in honey bee flight muscle.J. Musc. Res. Cell Motil. 1, 448.
BRADFORD, M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Analyt. Biochem. 72, 248–54.
BULLARD, B., BELL, J., CRAIG, R. & LEONARD, K. (1980) An actin-like protein from insect muscle.J. Musc. Res. Cell Motil,1, 194–5.
BULLARD, B., LUKE, B., & WINKELMAN, L. (1973) The paramyosin of insect flight muscle.J. molec. Biol. 359–67.
BULLARD, B. & REEDY, M. K. (1973) How many myosins per cross-bridge? II. Flight muscle myosin from the blowfly,Sarcophaga bullata.Cold Spring Harb. Symp. quant. Biol. 37, 423.
BULLARD, B. & SAINSBURY G. M. (1977) The proteins in the Z line of insect flight muscle.Biochem. J. 161, 399–403.
BUSCH, W. A., STROMER, M. H., GOLL, D. E. & SUZUKI, A. (1972) Ca2+-specific removal of Z lines from rabbit skeletal muscle.J. Cell Biol. 52, 367–81.
CHAPLAIN, R. A. & TREGEAR, R. T. (1966) The mass of myosin per cross-bridge in insect fibrillar flight muscle.J. molec. Biol. 21, 275–80.
DAYTON, W. R., REVILLE, W. J., GOLL, D. E. & STROMER, M. H. (1976) A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Partial characterization of the purified enzyme.Biochemistry 15, 2159–67.
EMES, C. H. & ROWE, A. J. (1978) Frictional properties and molecular weight of native and synthetic myosin filaments from vertebrate skeletal muscle.Biochim. Biophys. Acta 537, 125–44.
ETLINGER, J. D., ZAK, R. & FISCHMAN, D. A. (1976) Compositional studies of myofibrils from rabbit striated muscle.J. Cell Biol. 68, 123–41.
GOODE, M. D. (1972) Ultrastructure and contractile properties of isolated myofibrils and myofilaments fromDrosophila flight muscle.Trans. Am. Microsc. Soc. 91, 182–94.
GOLL, D. E., STROMER, M. H., ROBSON, R. M., LUKE, B. M. & HAMMOND, K. S. (1977) Asynchronous flight muscle. InInsect Flight Muscle (edited by TREGEAR, R. T.), pp, 15–40. Amsterdam: North Holland.
GREGORY, D. W. & PIRIE, B. J. S. (1973) Wetting agents for biological electron microscopy. I. General considerations and negative staining.J. Microsc. 99, 251–65.
HALL, C. E. (1958) Lengths of tobacco mosaic virus from electron microscopy.J. Am. Chem. Soc. 80, 2556–7.
HART, R. E. (1961) Surface structure of tobacco mosaic virus.J. molec. Biol 3, 701–2.
HPOLMES, K. C. (1980) Protein-RNA interactions during the assembly of tobacco mosaic virus.TIBS, Jan. 1980, 4–7.
JONES, A. V. & LEONARD, K. R. (1978) Scanning transmission electron microscopy of unstained biological sections.Nature 271, 659–60.
KING, L. & LEBERMAN, R. (1973) Derivatisation of carboxyl groups of tobacco mosaic virus with cystamine.Biochim. Biophys. Acta 322, 279–93.
KLUG, A. (1972) Assembly of tobacco mosaic virus.Fed. Proc. 31, 30–42.
KNIGHT, C. A. & WOODY, B. R. (1958) Phosphorus content of tobacco mosaic virus.Archs Biochem. Biophys 78, 460–67.
LAMVIK, M. K. (1978) Muscle thick filament mass measured by electron scattering.J. molec. Biol. 122, 55–68.
LEVINE, R. J. C., ELFVIN, M., DEWEY, M. M. & WALCOTT, B. (1976) Paramyosin in invertebrate muscles. II. Content in relation to structure and function.J. Cell Biol. 71, 273–9.
MAW, M. M. & ROWE, A. J. (1980) Fraying of A-filaments into three subfilaments.Nature 286, 412–4.
MILLMAN, B. M. & BENNETT, P. M. (1976) Structure of the cross-striated adductor muscle of the scallop.J. molec. Biol. 103, 439–67.
MORIMOTO, K. & HARRINGTON, W. F. (1973) Isolation and composition of thick filaments from rabbit skeletal muscle.J. molec. Biol. 77, 165–75.
MORIMOTO, K. & HARRINGTON W. F. (1974a) Substructure of the thick filament of vertebrate striated muscle.J. molec. Biol. 83, 83–97.
MORIMOTO, K. & HARRINGTON, W. F. (1974b) Evidence for structural changes in vertebrate thick filaments induced by calcium.J. molec. Biol. 88, 693–709.
PEPE, F. A. & DRUCKER, B. (1979) The myosin filament. VI. Myosin content.J. molec. Biol. 130, 379–93.
POTTER, J. D. (1974) The concent of troponin, tropomyosin, actin and myosin in rabbit skeletal muscle myofibrils.Archs Biochem. Biophys. 162, 436–41.
REEDY, M. K., ETLINGER, J. D., RABINOWITZ, M., FISCHMAN, D. A. & ZAK, R. (1975) Removal of Z-lines and α-actinin from isolated myofibrils by a calcium-activated neutral protease.J. biol. Chem. 250, 4278–84.
REEDY, M. K. (1968) Ultrastructure of insect flight muscle. I. Screw sense and structural grouping in the rigor cross-bridge lattice.J. molec. Biol. 31, 155–76.
REEDY, M. K., BAHR, G. F. & FISCHMAN, D. A. (1973) How many myosins per cross-bridge? I. Flight muscle myofibrils from the blowfly,Sarcophaga bullata.Cold Spring Harb. Symp. quant. Biol. 37, 397–422.
REEDY, M. K. & GARRETT, W. E. (1977) Electron microscope studies ofLethocerus flight muscle in rigor. InInsect Flight Muscle (edited by TREGEAR, R. T.), pp. 115–36. Amsterdam: North-Holland.
SQUIRE, J. M. (1973) General model of myosin filament structure. III. Molecular packing arrangements in myosin filaments.J. molec. Biol. 77, 291–323.
TREGEAR, R. T. & SQUIRE, J. M. (1973) Myosin content and filament structure in smooth and striated muscle.J. molec. Biol. 77, 279–290.
TRINICK, J. & ELLIOTT, A. (1979) Electron microscope studies of thick filaments from vertebrate skeletal muscle.J. molec. Biol. 131, 133–6.
TURNER, R. C., GREASER, M. L. & SCHULTZ, E. (1979) Association of myosin with myofibrils.Fed. Proc. 38, 339.
USALA, S., ISAACSON, M. & MARTIN, T. (1978) Scanning transmission electron microscopy of unstained nucleosomes.Proc. Ninth Int. Conf. Electron Microsc., Vol. II, pp. 224–225.
VALENTINE, R. C., SHAPIRO, B. M. & STADTMAN, E. R. (1968) Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme fromEscherichia coli.Biochemistry 7, 2143–52.
WEEDS, A. G. & POPE, B. (1977) Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility.J. molec. Biol. 111, 129–57.
WINKELHAHN, J. M. & BEINBRECH, G. (1974) Electron microscope studies on the dissociation of actomyosin by pyrophosphate.Experientia 30, 350–2.
WRAY, J. (1979) Filament geometry and the activation of insect flight muscles.Nature 280, 325–6.
ZEITLER, E. & BAHR, G. F. (1962) A photometric procedure for weight determination of submicroscopic particles, quantitative electron microscopy.J. appl. Phys. 33, 847–53.
ZIEGLER, A., HARRISON, S. C. & LEBERMAN, R. (1974) The minor proteins in tomato bushy stunt and turnip crinkle viruses.J. Virol. 59, 509–15.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reedy, M.K., Leonard, K.R., Freeman, R. et al. Thick myofilament mass determination by electron scattering measurements with the scanning transmission electron microscope. J Muscle Res Cell Motil 2, 45–64 (1981). https://doi.org/10.1007/BF00712061
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00712061