Abstract
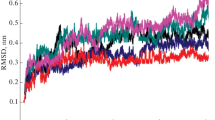
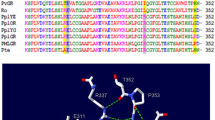
Luciferases are the enzymes that catalyze the reactions that produce light in bioluminescence. The bioluminescence color of firefly luciferases is determined by the luciferase structure and assay conditions. Amongst different beetle luciferases, those from phrixothrix rail-road worm with a unique additional residue (Arg353) emit red bioluminescence color naturally. Insertion of Arg356 in Lampyris turkestanicus luciferase changed the emitted light to red with a bimodal bioluminescence spectrum. By insertion and substitution of positively-charged residues, different specific mutation (E354R/Arg356, E354K/Arg356, E354R, E354K) lead to changes of the bioluminescence color. Bioluminescence emission spectra indicate that substitution of E354 by R along with insertion of Arg356 produces a luciferase that emits red light with a single peak bioluminescence spectrum. The comparison of mutants with native luciferase shows that mutations of firefly luciferase resulted in structural and functional thermostability. Comparative study of native and mutant luciferase (E354R/Arg356) by intrinsic and extrinsic fluorescence, CD spectropolarimetry, and homology modeling revealed mutation brought about an increase in content of secondary structure and globular compactness of L. turkestanicus luciferase. On the other hand, pKa of amino acids in the flexible loop decreased upon introducing of positive charges.
Similar content being viewed by others
Abbreviations
- SOE-PCR:
-
Splicing overlap extension-polymerase chain reaction
- PhRE:
-
Phrixothrix hirtus red light emitter
- Mg2+:
-
Magnesium
- rLuc:
-
Recombinant native luciferase
- Arg356:
-
Recombinant mutant luciferase containing Arg insertion
- BL:
-
Bioluminescence
- CD:
-
Circular dichroism
- RLU:
-
Relative light unit
- ANS:
-
8-Anilino-naphthalene-1-sulfonic acid
- Ni-NTA:
-
Nickel nitrilotriacetic acid
References
M. Deluca and W. D. McElroy, Purification and properties of firefly luciferase, Methods Enzymol., 1978, 57, 3–15.
H. H. Seliger and W. D. McElroy, Quantum yield in the oxidation of firefly luciferin, Biochem. Biophys. Res. Commun., 1959, 1, 21–24.
H. H. Seliger and W. D. McElroy, Spectral emission and quantum yield of firefly bioluminescence, Arch. Biochem. Biophys., 1960, 88, 136–141.
W. H. Biggley, H. J. E. Lloyd and H. H. Seliger, The Spectral Distribution of Firefly Light, J. Gen. Physiol., 1967, 50, 1681–1692.
N. P. Colepicolo, C. Costa and E. J. Bechara., Brazilian species of elaterid luminescent beetles. Luciferin identification and bioluminescence spectra, Insect Biochem., 1986, 16, 803–810.
V. R. Viviani and E. J. Bechara., Biophysical and biochemical aspects of phengodid (railroad-worm) bioluminescence, Photochem. Photobiol., 1993, 58, 615–622.
V. R. Viviani and E. J. Bechara., Bioluminescence of Brazilian fireflies (Coleoptera: Lampyridae): spectral distribution and pH effect on luciferase-elicited colors. Comparison with elaterid and phengodid luciferases, Photochem. Photobiol., 1995, 62, 490–495.
T. Arslan, S. V. Mamaev, N. V. Mamaeva and S. M. Hecht, Structurally Modified Firefly Luciferase. Effects of Amino Acid Substitution at Position 286, J. Am. Chem. Soc., 1997, 119, 10877–10887.
N. Kajiyama and E. Nakano, Isolation and characterization of mutants of firefly luciferase which produce different colors of light, Protein Eng., Des. Sel., 1991, 4, 691–693.
N. k. Tafreshi, M. Sadeghizadeh, R. Emamzadeh, B. Ranjbar, H. Naderi-Manesh and S. Hosseinkhani, Site-directed mutagenesis of firefly luciferase: Implication of conserved residue(s) in bioluminescence emission spectra among firefly luciferases, Biochem. J., 2008, 412, 27–33.
K. V. Wood, A. Y. Lam, H. H. Seliger and W. D. McElroy, Complementary DNA coding click beetle luciferases can elicit bioluminescence of different colors, Science, 1989, 244, 700–702.
Y. Ohmiya, T. Hirano and M. Ohashi, The structural origin of the color differences in the bioluminescence of firefly luciferase, FEBS Lett., 1996, 384, 83–86.
V. R. Viviani, A. J. S. Neto and Y. Ohmiya, The influence of the region between residues 220 and 344 and beyond in Phrixotrix railroad worm luciferases green and red bioluminescence, Protein Eng., Des. Sel., 2004, 17, 113–117.
N. N. Ugarova and L. Y. Brovko, Relationship between the structure of the protein globule and bioluminescence spectra of firefly luciferase, Russ. Chem. Bull., 2001, 50, 1752–1761.
M. Deluca, Hydrophobic nature of the active site of firefly luciferase, Biochemistry, 1969, 8, 160–166.
E. H. White and B. Branchini, Modification of firefly luciferase with a luciferin analog. A red light producing enzyme, J. Am. Chem. Soc., 1975, 97, 1243–1245.
F. McCapra, D. J. Gilfoyle, D. W. Young, N. J. Church and P. Spencer, in Bioluminescence and Chemiluminescence, Fundamental and Applied Aspects, ed. A. K. Campbell, L. J. Kricka and P. E. Stanley, 1994, Wiley, Chichester, pp. 387–391.
B. R. Branchini, T. L. Southworth, M. H. Murtiashaw, R. A. Magyar, S. A. Gonzalez, M. C. Ruggiero and J. G. Stroh, An alternative mechanism of bioluminescence color determination in firefly luciferase, Biochemistry, 2004, 43, 7255–7262.
E. Conti, N. P. Franks and P. Brick, Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes, Structure, 1996, 4, 287–298.
T. Nakatsu, S. Ichiyama, J. Hiratake, A. Saldanha, N. Kobashi, K. Sakata and H. Kato, Structural basis for the spectral difference in luciferase bioluminescence, Nature, 2006, 440, 372–376.
A. Lundin, Optimized assay of firefly luciferase with stale light emission, in Bioluminescence and Chemiluminescence: Status Report, ed. A. Szalay, L.J. Kricka and P. E. Stanley, 1993, John Wiley, Chichester, UK, pp. 291–295.
S. J. Gould and S. Subramani, firefly luciferase as a tool in molecular and cell biology, Anal. Biochem., 1988, 175, 5–13.
C. H. Contag and M. H. Bachmann, Advances in in vivo bioluminescence imaging of gene expression, Annu. Rev. Biomed. Eng., 2002, 4, 235–260.
T. C. Doyle, S. M. Burns and C. h. Contag, In vivo Bioluminescence imaging for integrated studies of infection, Cell. Microbiol., 2004, 6, 303–317.
A. Roda, P. Pasini, M. Mirasoli, E. Michelini and M. Guardigli, Biotechnological application of bioluminescence, Trends Biotechnol., 2004, 22, 295–303.
B. R. Branchini, T. R. Southworth, N. F. Khattak, E. Michelini and A. Roda, Red and green emitting firefly luciferase mutants for bioluminescence reporter application, Anal. Biochem., 2005, 345, 140–148.
P. J. White, D. J. Squirrell, P. Arnaud, C. R. Lowe and J. A. Murray, Improved thermostability of the North American firefly luciferase: saturation mutagenesis at position 354, Biochem. J., 1996, 319, 343–350.
H. H. Seliger and W. D. McElroy, The colors of firefly bioluminescence: enzyme configuration and species specificity, Proc. Natl. Acad. Sci. U. S. A., 1964, 52, 75–81.
N. Kajiyama and E. Nakano, Thermostabilization of firefly luciferase by a single amino acid substitution at position 217, Biochemistry, 1993, 32, 13795–13799.
L. C. Tisi, P. J. White, D. J. Squirrell, P. Arnaud, C. R. Lowe and J. A. Murray, Development of a thermostability firefly luciferase, Anal. Chim. Acta, 2002, 457, 115–123.
N. K. Tafreshi, S. Hosseinkhani, M. Sadeghizadeh, M. Sadeghi, B. Ranjbar, H. Naderi-Manesh, The influence of insertion of a critical residue (Arg356) in structure and bioluminescence spectra of firefly luciferase, J. Biol. Chem., 2007, 282, 8641–8647.
R. M. Horton, H. d. Hun, S. N. Ho, J. K. Pullen and L. R. Pease, Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension, Gene, 1989, 77, 61–68.
B. S. Alipour, S. Hosseinkhani, M. Nikkhah, H. Naderi-Manesh, M. J. Chaichi and S. Kazempour, Molecular cloning, sequence analysis, and expression of a cDNA encoding the luciferase from the glow-worm Lampyris turkestanicus, Biochem. Biophys. Res. Commun., 2004, 325, 215–222.
M. M. Bradford, A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, 72, 248–254.
S. Hosseinkhani, R. Szittner, M. Nemat-Gorgani and E. A. Meighen, Adsorptive immobilization of bacterial luciferases on alkyl-substituted Sepharose 4B, Enzyme Microb. Technol., 2003, 321, 86–93.
M. R. Eftink and C. A. Ghiron, Exposure of tryptophanyl residues and protein dynamics, Biochemistry, 1977, 16, 5546–5551.
M. Mehrabi, S. Hosseinkhani and S. Ghobadi, Stabilization of firefly luciferase against thermal stress by osmolytes, Int. J. Biol. Macromol., 2008, 43, 187–91.
J. Y. Cassim and J. T. Yang, A computerized calibration of the circular dichromate, Biochemistry, 1969, 8, 1947–1951.
T. Schwede, J. Kopp, N. Guex and M. C. Peitsch, SWISS-MODEL: an automated protein homology-modeling server, Nucleic Acids Res., 2003, 31, 3381–3385.
S. H. Northrup, MacroDox, v.2.0.2, Software for the prediction of macromolecular interaction, Tennessee Technological University, Cookeville, TN, 1995.
G. Vriend, WHAT IF: A molecular modeling and drug design program, J. Mol. Graph., 1990, 8, 52–56.
A. Riahi Madvar, S. Hosseinkhani, K. Khajeh, B. Ranjabr and A. Asoodeh, Implication of a critical residue (Glu175) in structure and function of bacterial luciferase, FEBS Lett., 2005, 579, 4701–4706.
J. R. Lakowicz and G. Weber, Quenching of fluorescence by oxygen. A probe for Structural fluctuations in macromolecules, Biochemistry, 1973, 12, 4171–4179.
A. Moradi, S. Hosseinkhani, H. Naderi-Manesh, M. Sadeghizadeh and B. S. Alipour, Effect of Charge Distribution in a Flexible Loop on the Bioluminescence Color of Firefly Luciferases, Biochemistry, 2009, 48, 575–582.
A. Kitayama, H. Yoshizaki, Y. Ohmiya, H. Ueda and T. Nagamune, Creation of a thermostable firefly luciferase with a pH-insensitive luminescence colour, Photochem. Photobiol., 2003, 77, 333–338.
Y. Ando, K. Niwa, N. Yamayda, T. Enomoto, T. Irie, H. Kubota, Y. Ohmiya and H. Akiyama, Firefly bioluminescence quantum yield and color change by pH- sensitive green emission, Nat. Photonics, 2008, 2, 44–47.
V. Viviani, A. Uchida, N. Suenaga, M. Ryufuka and Y. Ohmiya, Thr 226 is a key residue for bioluminescence spectra determination in beetle luciferases, Biochem. Biophys. Res. Commun., 2001, 280, 1286–1291.
S. Hosseinkhani, R. Szittner and E. A. Meighen, Random mutagenesis of bacterial luciferase: critical role of Glu175 in the control of luminescence decay, Biochem. J., 2005, 385, 575–580.
B. Baggett, R. Roy, S. Momen, S. Morgan, L. Tisi, D. Morse and R. J. Gillies, Thermostability of firefly luciferases affects efficiency of detection by in vivo bioluminescence, Mol. Imaging, 2004, 3, 324–332.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alipour, B.S., Hosseinkhani, S., Ardestani, S.K. et al. The effective role of positive charge saturation in bioluminescence color and thermostability of firefly luciferase. Photochem Photobiol Sci 8, 847–855 (2009). https://doi.org/10.1039/b901938c
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/b901938c